Treatment of Dementia-Associated Agitation With Gabapentin
Abstract
METHODS
CASE STUDIES
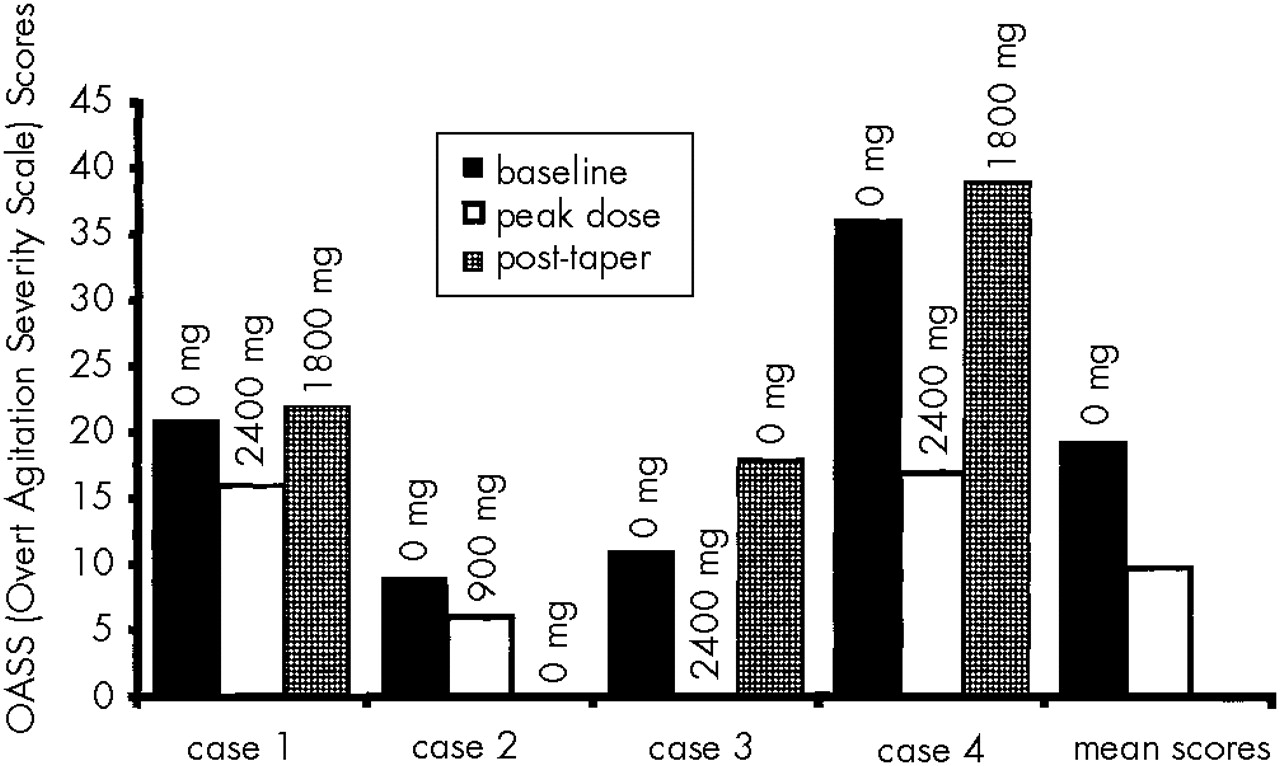
Case 1. A 73-year-old married man developed ataxia, hallucinations, memory loss, and hostile behavior about 1 year prior to participation in the study. According to his wife, the patient had a previous history of “mini-strokes.” On evaluation, he showed moderate agitation and voiced suspicion of his wife's infidelity. He had a 20-year history of alcohol abuse, but reportedly had not been drinking alcohol for 3 years prior to the study. He had no previous exposure to psychiatric medication. His baseline MMSE and OASS scores were 26 and 21, respectively. An MRI showed lacunar infarcts and patchy areas of hyperintensity in the periventricular white matter bilaterally. The patient was diagnosed with mixed dementia. He was started on 300 mg/day of gabapentin, which was titrated to 2,400 mg/day over 35 days. He complained of headaches and dizziness, which eventually remitted. He became calmer and more alert. The OASS score declined to 16, and the MMSE score remained at 26. When gabapentin was tapered to a daily dose of 1,800 mg, the patient's agitation increased and his OASS score increased to 22. Gabapentin was increased to 2,400 mg/day. The patient and his family were pleased with the effect of the medication and chose to continue at this dose following completion of the study.
Case 2. An 80-year-old married woman with a history of cognitive decline presented with difficulty performing simple tasks, episodes of nighttime agitation, and visual hallucinations. She had previously been treated with methylphenidate 5 mg/day for depression; this was discontinued prior to evaluation for study entrance. At the time of enrollment, she had been receiving donepezil 10 mg daily for Alzheimer's dementia for more than 6 months. On evaluation, the patient had impaired gait and severe memory impairment. Her MMSE score was 15 and her OASS score was 9. A CT scan revealed moderate cortical atrophy. The patient was started on 300 mg/day of gabapentin titrated to 900 mg over 3 days. Episodes of agitation decreased in frequency, and her OASS score fell to 6. However, she developed apparent side effects including sedation, fatigue, apathy, and decreased appetite, and her MMSE score dropped to 11. After 2 weeks on 900 mg/day, the dosage of gabapentin was reduced gradually to 100 mg tid. At this dose, the patient was brighter and more alert and regained her appetite. Her OASS score was 3 and her MMSE score was 17. Only one further episode of agitation was reported. After gabapentin was discontinued, the caregiver reported that the patient had continued improvement in behavior, and her OASS score fell to 0.
Case 3. An 80-year-old married woman with a history of memory loss and paranoia, diagnosed with Alzheimer's dementia, presented with episodes of agitation and depressed mood. She had been receiving donepezil 5 mg/day for 7 weeks. She accused family members of “freeloading.” A previous MRI had revealed mild atrophy and minimal bilateral periventricular white matter ischemic changes. The patient had no history of receiving psychiatric medications. Her baseline MMSE and OASS scores were 23 and 11, respectively. The patient was started on 300 mg/day of gabapentin, increased to a peak dose of 2,400 mg/day over 36 days. She developed daytime drowsiness and nighttime insomnia, but her agitation fully remitted. Her MMSE score was 21 and her OASS score was 0. When the medication was discontinued, depression, perseverative behaviors, and agitation returned. Her appetite and attention span decreased. Her final MMSE and OASS scores were 27 and 18, respectively. Gabapentin was restarted at 1,800 mg/day and the patient showed immediate improvement. Daytime sedation did not recur.
Case 4. An 87-year-old woman presented with memory impairment, depression, and agitation associated with probable Alzheimer's-type dementia. She had previous medication trials with valproic acid and paroxetine. During the week prior to enrollment she was tapered off of lithium 150 mg bid, trazodone 50 mg hs, and nortryptiline 50 mg hs. These medications had been ineffective. At the time of enrollment, she had been receiving donepezil 5 mg/day for more than 6 months. The patient was argumentative and combative toward her daughter and home attendants. A CT scan revealed moderate cerebral and cerebellar atrophy. Her baseline MMSE score was 24 and baseline OASS score was 36. The patient was started on 300 mg tid of gabapentin, increased to 2,400 mg/day over 33 days. Her MMSE and OASS scores at this dose were 26 and 17, respectively, indicating an improvement in agitation. The medication did not produce adverse effects. When the dose was lowered to 1,800 mg/day, the caregiver reported that the patient became more irritable, and her OASS score rose to 39. The gabapentin taper was halted. At a follow-up visit 6 months later, the patient was receiving 2,100 mg/day of gabapentin and had an OASS score of 11.
DISCUSSION
ACKNOWLEDGMENTS
References
Information & Authors
Information
Published In
History
Authors
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBGet Access
Login options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

