The efficacy of electroconvulsive therapy (ECT) in major depression is well established. Increasingly, patients with major depression receive ECT after failing to respond to one or more adequate antidepressant medication trials during the index episode.
1 It is common clinical practice to discontinue antidepressant agents before ECT, although this is not always possible. Therefore, except for a few well-defined interactions, remarkably little is known about the synergy or antagonism, or the benefits or risks, of the combined use of ECT and antidepressant drugs.
2–6In relation to efficacy, some early reports suggested that ECT appears not to be affected negatively or positively by concurrent tricyclic antidepressant (TCA) therapy.
7 In a retrospective study, Nelson and Benjamin
8 have reported better outcomes in patients receiving combination ECT-TCA than ECT alone. Moreover, in a recent report, rates of survival without relapse or recurrence for patients with major depression who received maintenance ECT and long-term antidepressant treatment were nearly doubled.
9 Thus, it would be helpful to learn more about ECT-antidepressant combinations through studies to determine their efficacy and potential or actual risks.
In recent years a series of new antidepressants has been introduced in clinical practice. Although having a similar pharmacological mechanism of action to TCAs, some of them are devoid of the anticholinergic side effects frequently associated with the tricyclics. Venlafaxine is a clinically effective antidepressant that, like the tricyclics, inhibits the neuronal reuptake of serotonin and norepinephrine,
10 but it has little effect on other neurotransmitter systems. It is well tolerated and has been proven to be effective for the management of patients with treatment-resistant major depression.
11Some cardiovascular complications can occur during ECT treatment in depressed patients with cardiac disease,
12–14 and also a mild incidence of cardiovascular adverse effects has been described with venlafaxine at dosages greater than 300 mg/day, including widening of the QRS interval.
15 Thus, it was considered interesting to study the association of venlafaxine with ECT during the treatment of refractory depression and to estimate its efficacy and safety.
METHODS
We retrospectively reviewed the records of patients with major depression, melancholic subtype (DSM-IV) who were hospitalized from January 1999 to December 1999 in the psychiatric ward of a general hospital because of treatment-resistant depression. All the selected patients had previously received a clinical trial of a tricyclic or tetracyclic antidepressant (imipramine, amitriptyline, maprotiline, or clomipramine), and a clinical trial of a selective serotonin reuptake inhibitor (paroxetine, fluoxetine or citalopram) during at least 6 weeks.
Among these patients, we analyzed the charts of patients who received venlafaxine at admission. The sample included 13 patients who were treated with venlafaxine in doses between 150 mg and 375 mg and who, during hospitalization for a period ranging from 1 to 2 weeks, received ECT because of extremely severe depressive symptoms. Of these 13 patients, 3 had a high risk of suicide, 7 had severe psychomotor symptoms, and 3 had both complications.
The patients and their families received written and oral information about electroconvulsive therapy, its risks and benefits, and ECT was given after obtaining their written informed consent. ECT was administered during a period of 3 to 4 weeks, following the American Psychiatric Association's recommendations.
16 The number of sessions per patient varied from 6 to 12. Treatment was given with bifrontotemporal electrode placement, brief pulse wave, in the surgery room, with atropine, propofol, and succinylcholine given immediately before ECT (MECTA SR1).
None of the patients had a history of previous cardiovascular disease. Thoracic X-radiography, electrocardiography, and laboratory testing were normal in all of the sample before ECT. Venlafaxine treatment was continued during ECT because of the extremely severe depression of patients, as it was considered a potential risk to withdraw the antidepressant. The doses of venlafaxine were not modified during the ECT treatment.
All of the patients were evaluated with the Hamilton Rating Scale for Depression (Ham-D, 21 items) and Clinical Global Impression (CGI) severity subscale the day before the first electroconvulsive treatment, and with the Ham-D Scale and CGI improvement subscale on day 28. Response was defined as a decrease on the Ham-D score of more than 50% from baseline to the 28th day, together with a CGI improvement score of 1 (very much improved) or 2 (much improved).
Statistical analysis was performed by using SPSS software and included descriptive measures and the Wilcoxon rank-sum test to compare depressive symptoms at admission and on day 28, and the Mann-Whitney U-test to compare doses of venlafaxine between groups. Statistical significance levels were set at P<0.05.
RESULTS
Of the 13 patients, 10 had unipolar and 3 bipolar depression.
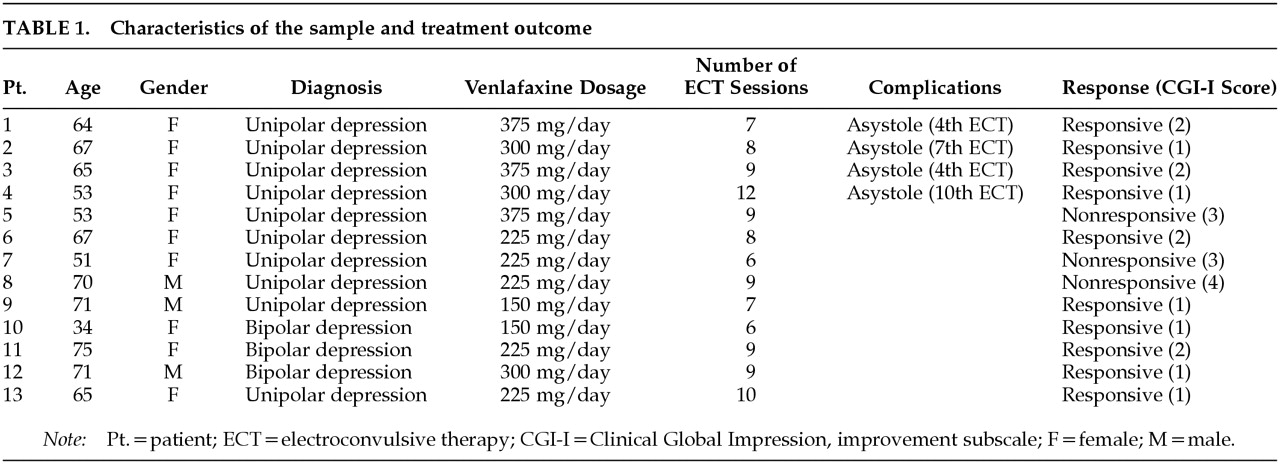
Table 1 displays some clinical features of the sample. The mean age was 62±11.30 years (range 34–75; means and standard deviations are reported). The sample included 10 women and 3 men.
The mean number of ECT sessions given across the study (28 days) was 8.38±1.66 (range 6–12). The mean daily dose of venlafaxine was 265.38±78.75 (range 150–375 mg).
The mean Ham-D score was 35.84±3.07 (range 32–42) before treatment and 15.30±11.39 (range 6–36) at day 28. There were significant differences between the scores at admission and at day 28 (
z=–2.87,
P<0.004). Ten of the 13 patients (76.9%) were considered to have responded to the treatment. (
Table 1)
A rapid reduction in heart rate followed by an asystole was observed in 4 sessions out of a total of 110, occurring in 4 different subjects. After atropine treatment, normal sinus rhythm returned. The other patients did not present cardiovascular complications. The patients with asystole had received significantly higher doses of venlafaxine (mean=337.5±43.30 mg, range 300–375) than patients without asystole (mean=265.38±78.75 mg, range 150–375; U=4, P=0.024).
There were no other complications such as prolonged seizures.
DISCUSSION
The results of this study show that combined ECT-venlafaxine was effective in a large percentage of these especially difficult-to-treat patients with depression. Positive responses were not associated with venlafaxine doses. Patients with relatively low doses (150–225 mg/day) were responsive to the treatment combination as well as patients who received relatively high doses (300–375 mg/day). Only 2 patients with venlafaxine doses lower than 300 mg/day were nonresponsive, and 1 patient treated with doses up to 300 mg/day did not respond to the treatment.
In terms of safety, however, the doses of venlafaxine used seem to be important. We found 4 cases of asystole, all of them in patients treated with doses of venlafaxine equal to or higher than 300 mg/day (1 patient with bipolar depression treated with 300 mg/day and 1 patient with unipolar depression treated with 375 mg did not have asystole). Therefore, although the small number of patients included in our study is a major limitation, the risk of asystole when ECT is associated with high doses venlafaxine cannot be ruled out.
To our knowledge, this is the first case series of high doses of venlafaxine combined with ECT in which asystole was an adverse event. There is a previous report of 1 patient with asystole during combined venlafaxine-ECT therapy.
17 However, recently a case series has been described of 9 patients who received low doses of venlafaxine (150 mg/day) and ECT and who did not experience cardiovascular complications.
18Temporary arrhythmias and repolarization abnormalities can occur in cardiologically healthy subjects and may be physiological side effects of ECT,
13,19 although asystole secondary to ECT is a possible, but rare, phenomenon. Some pharmacological actions of venlafaxine at high doses could contribute to explaining the cases of asystole observed; it would be worth considering these before combining ECT and venlafaxine at high doses.
Recently, it has been shown that venlafaxine blocks the fast inward sodium current (INa) of isolated guinea pig ventricular myocytes following its binding to the resting state of the channel,
20 the characteristics of blockage being different from those usually observed with most tricyclic antidepressants. Although an extrapolation of data obtained from animal experiments to humans must be made with care, this effect of venlafaxine could explain QRS prolongation and proarrhythmia observed in some patients with an increased plasma concentration of the drug (≥3×10
–6 M).
21 At therapeutic dosages, peak plasma concentrations of venlafaxine vary between 2×10
–7 M and 10
–6 M.
22,23 Consequently, blockage of sodium current could be observed in the upper range of these concentrations because 25% block of INa was measured at a concentration of 10
–6 M in guinea pigs.
21 Therefore, the blockage of sodium channels induced by venlafaxine at high doses in addition to the possibility of arrhythmias and repolarization abnormalities induced by ECT could account for the asystole episodes observed in our study. On the other hand, we used propofol as an anesthetic, and although neither pharmacodynamic nor pharmacokinetic interactions between venlafaxine and propofol have been reported, this anesthetic is able to block sodium currents in rat cardiac myocytes at concentrations comparable to those that may be attained during anesthesia.
24 Thus, a synergic or additive effect is possible when we associate high doses of venlafaxine with propofol. Interestingly, Agelink et al.
17 (who observed asystole) also used propofol as an anesthetic, whereas Bernardo et al.
18 (who did not observe asystole in their patients) did not.
A third, although remote, possibility is an interaction between propofol, succinylcholine, and the opioid system. In fact, a recent report showed that some actions of venlafaxine in mice can be antagonized by the opiate antagonist naloxone,
25 and asystole has been documented after the combination of propofol, succinylcholine (succinylcholine is also used in our anesthetic protocol), and fentanyl, an opiate analgesic.
26In summary, venlafaxine-ECT in combination seems to be effective in resistant-depression. Nevertheless, clinicians should be alerted to the possibility of asystole when patients are receiving high doses of venlafaxine and propofol is used as an anesthetic.
ACKNOWLEDGMENTS
The authors thank the medical staff of the Anesthetics Department for their participation in the treatment of the patients.