The Research Committee of the American Neuropsychiatric Association has chosen the subject of executive control function (ECF) for this report because of its impression that ECF is vital to human autonomy and a major determinant of problem behavior and disability in neuropsychiatric disorders. The core of this review is based on a literature search conducted in the spring of 1998. It was the Committee's intention to examine factor analyses of putative executive measures, community-based epidemiological studies of the prevalence of ECF impairment, and placebo-controlled clinical trials with executive outcome measures. All English-language articles and reviews published after 1966 that contained the keywords “frontal” or “executive” and were listed in the MEDLINE, EMBASE, PsychLit, or PsycINFO databases were considered. These articles were then separately cross-indexed with the keywords “controlled” (including both “placebo controlled” and “controlled clinical trial” subheadings), “prevalence,” and “factors.” Broad terms were used because of our impression that few data would be available at this stage in the literature's development. Peer-reviewed articles were retained. As we expected, very few relevant articles were identified. However, the original search was then further supplemented by backtracking to original sources and scholarly reviews of related topics. In addition, the original computer search strategy was repeated in January 2001 to take advantage of the exponentially increasing volume of research in this area.
In this review, we hope to provide a comprehensive, albeit still superficial, overview of the progress in ECF assessment. This concept is rapidly evolving across a wide range of disciplines. We first discuss the history of ECF and review its anatomical substrates. Then we address the obstacles to defining an executive “gold standard.” Next we examine recent functional neuroimaging studies. These have raised important questions about the localization of executive processes. We explore the relevance of ECF to various neuropsychiatric disorders. ECF may be particularly relevant to disability and problem behavior. Finally, we examine the possibilities for treatment of ECF impairment and suggest an agenda for future research.
HISTORICAL BACKGROUND
The “executive functions” broadly encompass a set of cognitive skills that are responsible for the planning, initiation, sequencing, and monitoring of complex goal-directed behavior. Although a coherent framework of executive control has yet to be developed, two central themes are emerging.
The first theme associates ECF with specific higher cognitive functions such as insight, will, abstraction, and judgment, which are mostly dependent on the frontal lobes.
1,2 This view implies that, like memory or language, the executive
cognitive functions are acquired skills that can be directly measured. ECF impairment results in the loss of these capacities.
The second theme emphasizes the cybernetic (from the Greek kybernetes, meaning “pilot”) aspects of executive function. Executive functions control the execution of complex activities. This view implies first that ECF interacts with nonexecutive processes, and second that ECF impairment is made visible only via the disorganized operations of nonexecutive domains. The cybernetic view of frontal function is not necessarily incompatible with the older emphasis on higher cognitive abilities, but it does bring a new emphasis on the dynamic interactions between frontal control systems and the processes they interact with.
The frontal lobes have been associated with the “higher” cognitive functions since at least the famous case of Phineas Gage.
3 However, the more limited sense of executive control has only recently emerged. This concept follows efforts to apply cybernetic principals to human behavior. For example, Miller et al. in 1960
4 applied the systems engineering concept of “TOTE” (Test Operate Test Exit) procedures to human cognition. Luria in 1969
5 initiated the modern era of clinical executive function assessment with his careful descriptive study of frontal head injuries among World War II veterans. In his book
The Working Brain (1973),
6 he described the clinical manifestations of disruption to a functional system for the “programming, regulation, and verification” of behavior. As early as 1977, Butterfield and Belmont
7 described executive function as the faculty in use “[when] a subject spontaneously changes a control process…as a reasonable response to an objective change in an information processing task” (p. 244). Norman and Shallice developed the concept of a “supervisory attentional system” in 1980.
8 This idea has been further refined into the “central executive,”
9,10 although the nature and functions of the central executive are still a matter of debate.
11–13 Clinicians soon associated frontal lobe injuries with the loss of behavioral regulation predicted by Shallice, Norman,
14,15 and Duncan.
16 Meanwhile, Marsden in 1982 pointed to the notable role of the basal ganglia in organizing and controlling motor actions.
17 Major advances followed the work of Alexander and colleagues.
18,19 Working with primates, they demonstrated that the frontal lobes were associated with distinct basal ganglia–thalamocortical circuits. Lesions to these circuits produce “frontal lobe” behavior and personality changes. Moreover, Goldman-Rakic and colleagues demonstrated that the effects of frontal cortical lesions can be reproduced all along the related circuit.
20–23 This research explained the appearance of “frontal” syndromes following subcortical lesions and greatly expanded the list of conditions that could potentially affect executive control.
In 1990, DeKosky and Scheff
24 identified mesiofrontal synaptic density as the strongest pathological determinant of dementia severity ratings that has yet been reported in Alzheimer's disease (AD). This finding opens up the possibility that frontal pathology, and by extension ECF impairment, may be the essential feature of dementia. Later studies have shown that only pathology in the frontal cortex (or select afferents) is both necessary and sufficient to explain the clinically recognized dementia in AD
25 and non-AD dementias.
26 Concurrent with these developments, researchers using functional imaging began to identify frontal metabolic deficits and correlate them with clinical pathology in conditions as diverse as schizophrenia, major depression, and attention-deficit/hyperactivity disorder (ADHD). These and other clinical correlations led, in 1994, to the inclusion of ECF in the American Psychiatric Association's definition of dementia.
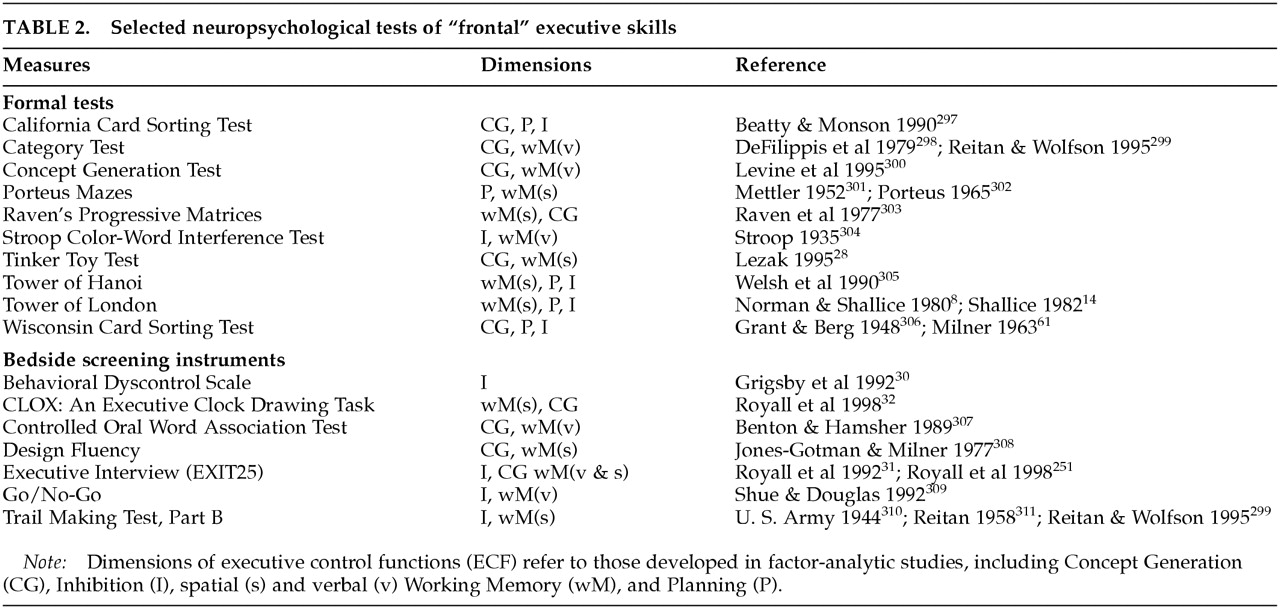
27 However, the clinical assessment of executive function has lagged behind these advances. This is partly because of the lack of suitable measures. The Stroop Color/Word Interference Test (Stroop), the Trail Making Test Part B (Trails B) of the Halstead-Reitan battery, the Conceptualization Task of the Dementia Rating Scale, and a variety of other tests of abstraction and mental control have been offered as putative ECF measures.
28 The Wisconsin Card Sorting Test (WCST) is perhaps the best described ECF test (see box, p. 391), but these and other formal executive measures are often impractical for widespread use outside of academic settings.
In 1990, Kaye et al. introduced the Behavioral Dyscontrol Scale (BDS), a brief compilation of clinical items adapted from the work of Luria.
29,30 In 1992, Royall et al. introduced the Executive Interview (EXIT25),
31 followed in 1998 by CLOX: An Executive Clock Drawing Task.
32 Most recently, the Frontal Assessment Battery (FAB)
33 has been introduced. This instrument is similar to the BDS and the EXIT25 in that it is a compilation of simple clinical ECF assessments. However, the FAB differs from earlier measures in that its item set was designed to elicit several distinct executive tasks, each of which can be significantly correlated with frontal metabolic changes.
Another approach to ECF assessment has been to identify the behavioral sequelae of executive dyscontrol and to measure these. Behavior rating scales, such as the Neuropsychiatric Inventory (NPI),
34 contain subtests for behaviors that have been specifically associated with frontal lesions. The Behavioural Assessment of the Dysexecutive Syndrome (BADS)
35 and the Frontal Lobe Personality Scale (FLOPS)
36 have been explicitly developed to measure “dysexecutive” behavior syndromes.
This new generation of ECF instruments can be administered by clinicians in almost any setting. Consequently, executive impairment has been demonstrated in almost every major neuropsychiatric disorder (reviewed below). In many of these conditions, measures of executive function are more strongly associated with functional status, level of care, and need for services than are either syndrome-specific positive symptoms (e.g., psychosis, mood disturbance, or memory loss) or nonexecutive cognitive domains.
ANATOMICAL SUBSTRATES OF ECF
The Prefrontal Cortex
The role of the prefrontal cortex in executive function is suggested by its unique structure and pattern of connectivity.
37 The prefrontal cortex (Brodmann areas [BA] 8–11, 24, 25, 32, 45–47) comprises more than 30% of the brain's weight and surface area. It is a phylogenetically recent structure, representing only 10% to 20% of the primate brain.
38 The frontal cortex can be grossly divided into two cytoarchitectural regions. The posterior portion is “agranular” in nature. This term refers to the minimal representation of the internal granular layer IV in posterior frontal cortical sections. In contrast, the regions that are most closely associated with executive function (e.g., the anterior [“prefrontal”] portion of the frontal lobes, which comprises the dorsolateral and orbital/medial regions) consist of “granular cortex.” This term refers to a cortical architecture in which layer IV is distinct and well developed. Layer IV is most developed in BA 46 and becomes progressively less distinct as one moves ventrally and posteriorly from there.
Cortical layer IV is rich in inhibitory GABAergic interneurons. These interneurons receive input from bioaminergic nuclei in the brainstem and “feed forward” to provide inhibition to local pyramidal cells in cortical layers III and V. GABAergic interneurons have been implicated in the executive impairments of schizophrenia
39 and may represent one of the principal targets of atypical neuroleptics.
Several unique aspects of the prefrontal cortex suggest that it mediates ECF. First, the prefrontal cortex is connected to more brain regions than any other cortical region. Only the primary sensorimotor cortices and subcortical sensorimotor relay nuclei do not have direct or simple indirect connections to the prefrontal cortex. Second, the frontal cortices are “metamodal”: they receive direct cortical input only from other heteromodal association areas. Thus, they are positioned to act on information that has already been processed at lower levels. The integrative nature of prefrontal regions is reflected even at the cellular level. Many frontal neurons increase their firing rate in response to the combined activity of sensory and motor regions. Additionally, frontal firing patterns may be altered by manipulating the motivational importance of environmental stimuli. Third, the prefrontal cortex is the major neocortical target for information processed in the limbic circuits. It is the only cortical region positioned to integrate cognitive and sensorimotor information with emotional valences and internal motivations. Fourth, although wide areas of the cortex project into the basal ganglia–thalamocortical circuits, the prefrontal cortex is that system's major target. Thus, the frontal lobe is the only cortical region capable of integrating motivational, mnemonic, emotional, somatosensory, and external sensory information into unified, goal-directed action.
In addition, the prefrontal cortex has bilateral connections to the basal ganglia–thalamocortical circuits' targets in the thalamus. Similarly, the prefrontal cortex has bilateral connections to its afferents in the parietal, temporal, and occipital association cortices, the limbic circuits, and the major brainstem biogenic aminergic nuclei, as well as to the cholinergic neurons of the nucleus basalis of Meynert. These connections put the prefrontal cortex in a unique position to modify the information it acts on. Moreover, in the case of the major brainstem bioaminergic nuclei, which project diffusely to the cortex, the prefrontal cortex is positioned to indirectly influence the activity of the nonfrontal cortex as well.
Frontal Basal Ganglia–Thalamocortical Circuits
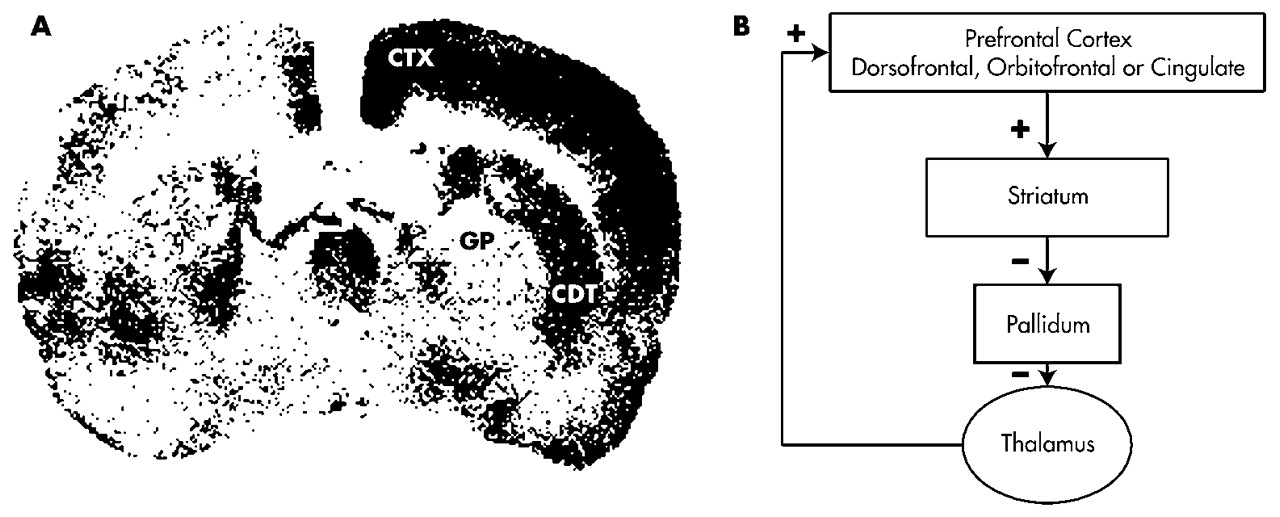
Certain subcortical lesions can affect ECF either directly or indirectly via frontal cortical metabolic changes (e.g., by diaschisis). The caudate, putamen, pallidum, nucleus accumbens, and thalamus are related to the frontal cortex through basal ganglia–thalamocortical behavioral control “circuits” (
Figure 1A).
19,40,41 Although each of these circuits passes through different structures, all of the frontal circuits are similar in design.
The neurochemistry of these circuits' connections is known.
42 Excitatory glutamatergic fibers from the cortex project to the neostriatum (caudate, putamen); then inhibitory GABAergic fibers project to the globus pallidus/substantia nigra and from there to specific targets in the thalamus. These connections form dynamically balanced direct and indirect circuits connecting the prefrontal cortex to the thalamus. The thalamus closes the circuit by projecting back to prefrontal cortical regions via stimulatory glutamatergic fibers. Cholinergic projections to the frontal cortex facilitate thalamic activation of that structure. Dopamine (DA) projections from the ventral tegmentum also innervate the cortex. DA projections from the nigra innervate the striatum.
In each circuit, the corresponding frontal cortical region and striatum receives inputs from cortical regions that are more posterior.
43–46 These inputs provide insights into each circuit's functional role by revealing the processes with which it interacts. The dorsofrontal circuit receives information from the parietal and temporal cortex. These regions provide access to complex spatial and temporal information. The orbitofrontal circuit receives input from visual and auditory processing areas in the occipital and temporal lobes, as well as limbic centers in the amygdala and temporal poles. The anterior cingulate/mesiofrontal cortex receives input from the hippocampus, amygdala, and paralimbic cortex. Some authors have labeled the anterior cingulate circuit “paralimbic” for this reason.
Several aspects of this circuitry also deserve special mention. First, these circuits funnel information from widespread cortical areas into relatively small thalamocortical targets. These targets are all in the prefrontal cortex, consistent with the role of these circuits in behavioral/cognitive control. Second, the behaviors that mark each circuit can be reproduced by lesions at various points along their path. For example, the ability to perform certain visuospatial “working memory” tasks (which involve the short-term maintenance of information during its manipulation) is dependent on the integrity of the dorsolateral prefrontal cortex.
23 However, the same tasks are disrupted by lesions to the caudate
20 and to the mediodorsal thalamic nucleus
21,22 in the dorsofrontal circuit. This association suggests that frontal cortical damage is a sufficient but not a necessary cause of executive dyscontrol. Finally, the circuits appear to be discrete (i.e., nonoverlapping) and spatially constrained. At the level of the cortex, they are widely separated. Cortical lesions can divorce the behaviors associated with one circuit from another. Subcortically, however, the circuits are in much closer proximity. This anatomy suggests that subcortical pathology is likely to lesion multiple circuits simultaneously, mixing the syndromes together.
Three frontal circuits are particularly relevant to executive control: the dorsolateral prefrontal circuit, the lateral orbitofrontal circuit, and the anterior cingulate circuit.
18,47 Dorsolateral Prefrontal Circuit:
The dorsolateral convexities of the frontal lobes consist of BA 8–12, 46, and 47. The blood supply for these regions is from the middle cerebral artery. In the dorsolateral circuit, corticofugal pathways project to the dorsolateral caudate nucleus, which also receives input from the posterior parietal cortex and the premotor area. The circuit then connects to the dorsolateral portion of the globus pallidus and the rostral substantia nigra reticulata and continues to the parvocellular region of the medial dorsal and ventral anterior thalamic nuclei. The circuit is closed via thalamic projections back to the frontal dorsolateral convexity. Lesions to this circuit have been implicated in a variety of higher cognitive functions, including goal selection, planning, sequencing, response set formation, set shifting, verbal and spatial working memory, self-monitoring, and self-awareness (metacognition).
38,48–52 The WCST consistently activates dorsolateral frontal regions.
Lateral Orbitofrontal Circuit:
The “orbit” of the frontal lobes refers to a continuous region including ventral anterior and inferior lateral regions (BA 10–15 and 47). Medial regions are vascularly supplied by the anterior cerebral artery, and lateral regions lie in the territory of the middle cerebral artery. Cortical projections terminate on the ventromedial caudate nucleus, which also receives input from other cortical association areas, including the superior temporal gyrus (auditory) and inferior temporal gyrus (visual), as well as brainstem regions (e.g., the reticular formation). Projections continue to the dorsomedial aspect of the internal globus pallidus and to the rostromedial portion of the substantia nigra reticulata. Pathways continue to the magnocellular region of the medial dorsal and ventral anterior thalamic nuclei, and then return to the lateral orbitofrontal region.
The orbitofrontal circuit appears to be involved in the initiation of social and internally driven behaviors and the inhibition of inappropriate behavioral responses.
48,52 Orbitofrontal function may be particularly relevant to risk assessment. Choosing between small but likely rewards and large yet unlikely rewards activates inferior and orbitofrontal regions.
53 Impairment on the “go/no-go” task has been associated with orbitofrontal lesions in animals
54 and humans.
55 Orbitofrontal lesions also lead to clinical features such as environmental dependency and utilization behavior.
56–58 Anterior Cingulate Circuit:
Frontal regions involved in this circuit are medially located (BA medial 9–13, 24, and 32), and receive their blood supply from the anterior cerebral artery. The circuit connects to the ventral striatum (nucleus accumbens and olfactory tubercle), which receives additional input from “paralimbic association” cortex, including anterior temporal pole, amygdala, inferior hippocampus, and entorhinal cortex. The circuit continues to the ventral pallidum and rostrodorsal substantia nigra, and then to the medial dorsal thalamic nucleus. It terminates at the anterior cingulate, completing the circuit.
The anterior cingulate is important in monitoring behavior and error correction. The Stroop activates the anterior cingulate and its mesiofrontal extensions.
59 The EXIT25
31 has also been specifically associated with left mesiofrontal cerebral blood flow by single-photon emission computed tomography (SPECT).
60 OBSTACLES TO DEFINING AN EXECUTIVE “GOLD STANDARD”
One of the obstacles to ECF research has been the lack of a clear “gold standard” measure against which putative ECF measures can be compared. This measure would presumably call upon specific frontal functions and be selectively vulnerable to frontal pathologies. However, this may not be an achievable goal for three reasons. First, since the frontal lobe represents so much of the brain's weight and surface area, it seems unlikely that any one measure could assess its functions comprehensively. We may be searching for a frontal-executive battery, not an executive measure. Second, the anatomy of frontal systems suggests that specific subcortical pathologies are also relevant to ECF. Thus, we may not even be looking for a frontal battery so much as a frontal system battery. Finally, the cybernetic character of ECF implies an intimate relationship between ECF and its associated targets. We will need to qualitatively distinguish between the loss of executive control over a nonexecutive domain and a primary disruption of the domain itself.
For example, although some tasks (e.g., the WCST, the Stroop, the Category Test, the EXIT25, and Trails B) have been specifically associated with frontal structural or metabolic changes,
61–66 they can also be affected by more posterior lesions.
67–70 WCST performance is not specific for frontal lobe damage unless deficits in comprehension or visual search are controlled.
71 Furthermore, both the WCST and the Stroop measure multiple dimensions of executive control in factor-analytic studies. These dimensions may not be localizable to the frontal lobes even if frontal systems are a major determinant of their variance.
Peterson et al.
59 provide an example of this problem for the Stroop. This measure activated multiple nonfrontal cortical regions, which in turn resolved themselves into seven discriminable factors. These factors were interpreted as representing distributed neuronal networks supporting error monitoring, working memory, selective attention, and motor planning (among others). Although several Stroop factors shared the anterior cingulate, cingulate activation does not uniquely explain Stroop variance, and many nonfrontal lesions have the potential to affect Stroop performance. Nevertheless, activation studies have been criticized for their sensitivity to “subclinical” differences in performance.
72 Frontal lesions selectively affect the Stroop in actual patients.
73 Thus, poor Stroop performance may yet be indicative of frontal pathology, despite the complexity of activation studies.
It appears that neither the measures used to assess ECF nor the biological substrates they activate are easily localizable. Four important dichotomies need to be addressed before these apparent discrepancies can be resolved. Each will be discussed in turn.
1.
Frontal Lobe vs. Frontal System: Frontal cortical lesions may be sufficient, but are not necessary causes of executive impairment.
2.
Structure vs. Function: Frontal cortical function may be compromised by subcortical lesions (i.e., vascular disease) in the absence of demonstrable local cortical pathology.
3.
Control vs. Process: Executive functions control performance in other neuropsychological domains. Some tasks that were previously ascribed to nonexecutive domains may be sensitive to frontal system pathology because they require executive control. Conversely, lesions outside the frontal systems may undermine ECF test performance, in the absence of executive dyscontrol, by disrupting the processes being controlled during the task.
4.
Executive Function vs. Executive Function(s): Some measures may be sensitive to only a subset of executive functions.
Frontal Lobe vs. Frontal System
It has proven difficult to localize specific executive operations to specific prefrontal regions. Rather, ECF may depend on the integrity of frontal systems. For example, L'Hermitte et al.
57 have described the phenomenon of “utilization behavior” (in which a patient automatically utilizes a familiar object in a habitual way, regardless of its appropriateness to the current context) following orbitofrontal lesions. The same behavior has been described following massive bilateral frontal lesions
74 and mesiofrontal lesions,
75 both of which might involve orbitofrontal regions. However, utilization behavior has also been reported following lesions to other frontal system structures, including the caudate
76 and thalamus.
77 The unity of frontal circuit activity can be deduced from factor analyses of regional brain metabolism: 70% of regional variance in total cerebral glucose utilization can be explained by a single factor that contains the frontal circuits (e.g., the frontal cortex, cingulate gyrus, caudate nucleus, putamen, and thalamus) and temporal cortex.
78 There may be several reasons for the difficulty in making clinicopathological correlations between ECF and frontal lesions: 1) the taxonomy of executive impairments has not been adequately developed—many authors may not be comparing identical phenomena; 2) although discrete prefrontal pathways have been partially established, precise anatomical boundaries are not well defined, especially at the cortical level, and certain frontal functions are limited to subregions of traditional BA regions of interest;
79 3) lesions to the frontal lobes are often not well defined or do not follow clear and reproducible boundaries across subjects (e.g., most frontal strokes cause additional damage to subcortical or posterior regions); 4) frontal lobe pathology, such as tumors, stroke, or trauma, frequently results in remote effects secondary to vascular changes, pressure effects, and disconnection of neural pathways. Data from psychosurgery (tumor evacuation or frontal leukotomy) can be especially difficult to interpret for several reasons: a) these studies often use abnormal patients to begin with, b) cognitive outcome assessment is often rudimentary, and c) follow-up is typically short term (i.e., months rather than years).
Nonetheless, it now appears that there are regional differences in behavioral sequelae of frontal cortical lesions.
5,38,80–82 Damage to the dorsolateral prefrontal cortex impairs planning, hypothesis generation, and behavioral control. Episodic memory encoding and retrieval is affected by ventrolateral lesions. Working memory is affected by more dorsal pathology. Orbitofrontal lesions lead to impaired insight, judgment, and impulse control. These traits were part of Phineas Gage's deterioration. Mesiofrontal/anterior cingulate lesions lead to indifference and attentional dyscontrol. Patients generate little speech or behavior spontaneously, yet may respond correctly if prompted.
Moreover, the dysexecutive neuropsychological profile of prefrontal
cortical disorders such as frontotemporal dementia can also be observed in
subcortical frontal system disorders such as Parkinson's disease (PD), Huntington's disease (HD), progressive supranuclear palsy,
83 or subcortical vasculopathy.
84 Even neuropsychiatric disorders such as major depression and schizophrenia are associated with a similar pattern on psychometric testing, suggesting that they too may involve frontal system pathology.
85,86 In summary, executive functions have been difficult to localize within the frontal cortex. This situation might be improved with more careful attention to lesion location and a formal approach to frontobehavioral nomenclature. Nonetheless, the logic of frontal basal ganglia–thalamocortical networks suggests that frontal system lesions are both sufficient and necessary to executive impairments.
Structure vs. Function
Another dichotomy that deserves attention is that between frontal structure and function. Executive control can be compromised without a frontal cortical lesion. Frontal function can be indirectly affected by lesions to frontal lobe afferents or related frontal system circuit structures. Conversely, lesions to corticofugal tracts can disconnect the frontal operations from the processes they control.
Human and animal studies suggest that subcortical lesions to frontal system networks may remotely affect frontal cortical metabolism (e.g., by diaschisis), either increasing or decreasing frontal metabolism.
Figure 1A presents the results of a study by Kelly and McCulloch
87 in which rats received a 500-ng injection of muscimol (a GABAergic agonist) to the left caudate nucleus. This lesion resulted in a functional caudate lesion on that side. The effects of this lesion were studied using [
14C]2-deoxyglucose autoradiography. Brain regions that were metabolically active at the time of injection took up this radioligand. Regions that were metabolically inactive, including the left caudate, did not take up the tracer. In each section, the right (unaffected) side served as the left's control.
Figure 1B demonstrates that the caudate lesion resulted in disinhibition of the ipsilateral globus pallidus, leading to increased inhibition of the ipsilateral medial thalamic nucleus, resulting in reduced activation of the ipsilateral cortex. In short, a discrete subcortical lesion in frontal networks may lead to remote changes in frontal cortical metabolic function. This finding can be understood in the context of frontal circuit anatomy (
Figure 1A) and may help to explain the finding of frontal behavioral syndromes and ECF impairment in subcortical dementias,
88 as well as the specific association between subcortical vasculopathy and frontal hypometabolism in vascular dementia (VaD) and late-onset major depression.
89–91 Patients with PD, major depression, and schizophrenia often appear “hypofrontal” by functional neuroimaging.
92–95 In PD and major depression, this may be related to cortical deafferentation of medial nigral or ventral tegmental DA inputs.
96 The hypofrontality of both disorders is associated with tests that are linked to DA physiology.
97,98 Alternatively, these deficits might be related to cortical deafferentation of the thalamic inputs.
19,90,99 Medial thalamic infarction results in frontal cortical hypometabolism by positron emission tomography (PET) and SPECT.
100,101 Thalamic outputs to the frontal cortex can be disrupted indirectly after globus pallidus lesions.
102 However, executive impairments are not only associated with frontal hypometabolism. In obsessive-compulsive disorder (OCD), cortical hypermetabolism
103 is associated with poor performance on ECF measures.
104 Similarly, in HD the degree of prefrontal activation during the WCST is inversely proportional to the subject's performance, yet is statistically associated with the amount of caudate atrophy.
105 These seemingly paradoxical findings may be understood from the point of view of frontal systems physiology. OCD has been associated with hypometabolism in the globus pallidus and thalamic disinhibition. Thalamic disinhibition might result in increased thalamocortical glutamatergic tone (
Figure 1A). Thalamocortical glutamatergic inputs co-localize with inhibitory dopamine D
1 receptors on pyramidal cell dendrites in the prefrontal cortex.
40 The balance between these opposing influences affects prefrontal signal-to-noise processing.
106 Either increasing glutamatergic excitation or diminishing dopaminergic pyramidal cell inhibition should lead to increased pyramidal cell activity, at the expense of signal specificity. A precise range of DA receptor activity within the prefrontal cortex must be maintained for optimal function.
107–108 In the case of OCD, DA's inhibitory effects may be overwhelmed by increased glutamatergic tone.
In summary, executive control depends on the integrity of frontal systems. Executive impairment may follow disruption of frontal system information processing, regardless of the location of the lesion within the system or the direction of the perturbation. In some cases, remote lesions can affect processing within the frontal circuits.
Control vs. Process
Lezak
28 has offered a simple test for defining what constitutes an “executive” measure. Questions about executive functions explain “
how or
whether a person goes about doing something…questions about [traditional] cognitive functions are generally phrased in terms of
what or
how much.” (p. 42). This simple dichotomy cleaves the vast array of frontal functions into control functions and their target processes. Either may be dependent on frontal activities; however, only the control functions are “executive” in a cybernetic sense. The subset of frontal functions that are “executive” depends on how the question is asked (
Table 1).
28 This distinction can be addressed experimentally. For example, there is an extensive literature associating schizophrenia with deficits on the WCST. However, patients with schizophrenia benefit from cueing during the WCST test procedure.
109 In other words, they can
generate the abstract concepts demanded by the task, but they do not
apply them unless prompted. Thus, although the abstract concept formation demanded by the WCST may in fact be localizable to the frontal lobes, it is not necessarily an executive control function in the limited sense required by Lezak because it merely addresses what patients
can do and not whether they do it when needed. In contrast, the failure of patients with schizophrenia to inhibit automatic but inappropriate verbal responses on tests such as the Stroop
110 would be more consistent with Lezak's view of executive control.
Authors who emphasize a cybernetic view of ECF point to the potential to observe executive
dyscontrol in performance on many seemingly “nonexecutive” tasks.
12,111 By analogy, at least some variance in all neuropsychometric tests may be specifically attributable to the executive control demanded by the testing paradigm (see “g” below). We will examine the executive control of clock drawing, memory, and language.
The clock-drawing task (CDT) has traditionally been viewed as a visuospatial task, sensitive to right hemisphere pathology.
112 However, frontal leukotomy selectively affects CDT performance relative to age, disease, and education-matched control subjects.
113 CDT failures among frontally impaired subjects challenge a chiefly visuospatial conceptualization of the CDT and suggest the need for a separate analysis of the executive control demanded by the testing paradigm.
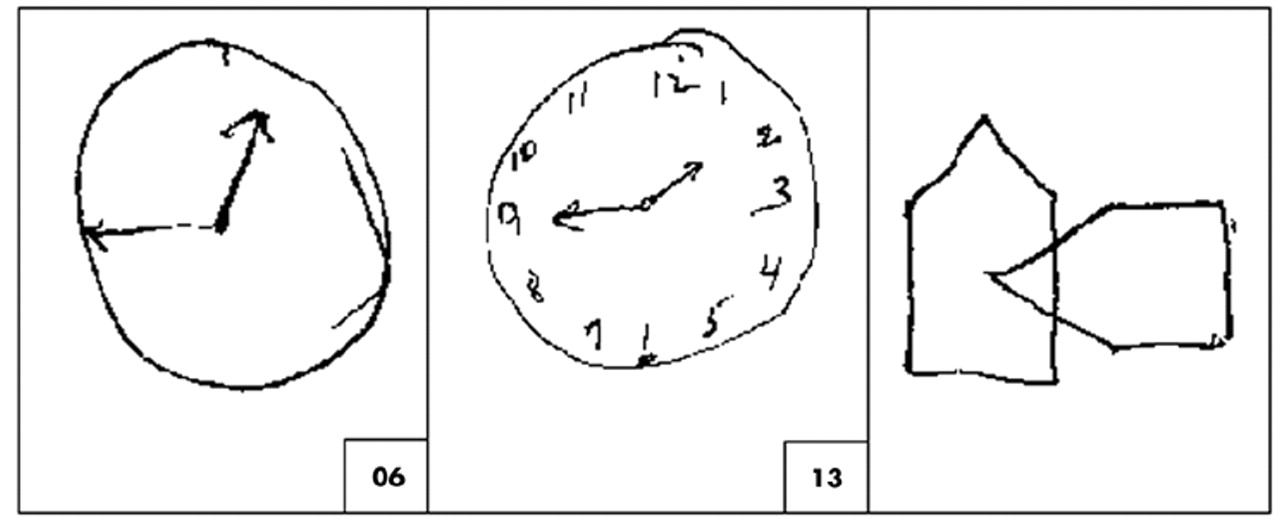
Figure 2 presents a patient's performances on CLOX, an executive CDT.
32 CLOX1 is an unprompted task. CLOX2 is a copied version. The visuospatial components of these tasks are similar. However, CLOX1 entails executive control because it requires the subject to generate a figure in the absence of relevant visual cues. The validity of CLOX1 as an executive paradigm is suggested by the fact that, in elderly retirees, both CLOX1 and the EXIT25, but neither CLOX2 nor the Mini-Mental State Examination (MMSE) makes significant independent contributions to the number of categories achieved on the WCST.
114 Figure 2 presents the pattern of CLOX performance expected in a frontal system disorder. Executive measures (the unprompted CLOX1 and the EXIT25) are impaired. CLOX2 (copied) and the MMSE are not.
The same qualitative dissociation between control and process can be elicited in other domains, such as memory. Memory tasks can be affected by frontal, parietal, and mesiotemporal cortical lesions. However, the pattern of memory loss that follows frontal system lesions is discriminable from traditional limbic amnesia.
115–119 The ability of a “memory” task to activate dorsofrontal systems depends greatly on the structure provided to the subject during memory testing.
120,121 For example, the intentional, goal-directed retrieval of information results in frontal activation relative to incidental cued recall.
122 Patients with frontal lesions are unimpaired in their ability to recall cued information, but have difficulty with tasks that require them to organize, sequence, or monitor the information themselves. Thus, they have trouble with free recall, temporal order, and source memory. Similarly, confabulation among amnestic subjects appears to reflect mesiofrontal/anterior cingulate impairment, resulting in a failure to ignore active but currently irrelevant memory traces.
123 Not all memory tasks that activate frontal regions are necessarily “executive.” In neuroimaging studies, tasks that call for relatively simple episodic or semantic encoding tend to activate the left ventrolateral prefrontal cortex.
124 Those that call for retrieval activate the right ventrolateral prefrontal cortex. However, if the subject is asked to manipulate the information while encoding or retrieving it, the focus of activation shifts toward more dorsolateral regions.
125 Language skills are also affected by ECF impairment. Arbuckle and Gold
126 have associated disorganized and hyperverbose speech, but not language impairment per se, with impaired working memory and executive control. Similarly, only a small amount (25%) of variance in verbal fluency scores can be explained in multivariate regression models by tests of verbal memory, verbal attention, and vocabulary.
127 The idea that ECF may explain some variance in most cognitive measures, regardless of the domains they purport to measure, is similar to Spearman's concept of “general intelligence” or “g.”
128 “g” represents the shared variance across domains and has been repeatedly observed in batteries of multiple cognitive measures. For example, in normal aging there are significant declines in cognitive test performance across several domains. Salthouse et al.
129 found moderate age-related declines on a battery of tests that included the WCST, Trail Making, Wechsler Adult Intelligence Scale–Revised (WAIS-R) Block Design, and Digit Symbol Substitution (DSS).
However, correlation-based analyses revealed that the age-related effects on different measures were not independent. Instead, the effect of age was observed specifically in the fraction of variance (averaging 58%)
shared across all measures (i.e., “g”); “g” has been localized to dorsolateral prefrontal cortex by PET
130 and associated with working memory (also associated with dorsolateral prefrontal cortex; see below)
131,132 and with formal executive measures.
133 In summary, there is no established framework for interpretation of the executive functions. Some authors emphasize the frontal lobes and their importance in planning, hypothesis generation, and abstraction. Others, however, work within a more limited subset of frontal functions. These authors see ECF as a specific subset of frontal lobe activities, revealed by the examination of how the frontal systems interact with other systems to produce and control complex goal-directed activities.
Executive Function vs. Executive “Function”
Another dichotomy that has yet to be resolved is whether there is a single executive control, as opposed to multiple controls for discrete operations. The idea of a single executive is implied in the concept of the “central executive” and the multimodal nature of the frontal lobe's anatomy and functional connections. Researchers have developed computer models of subject task performance on putative “frontal” measures that successfully model patient task performance on four frontal tasks (the WCST, the Stroop task, motor sequencing, and a context-dependent memory task).
134 Frontal-type errors on all tasks can be observed after degrading a single domain (working memory).
However, patients with frontal lesions often display disassociations in their performance on select frontal tasks. This effect might be due to regional differences in the types of processes to which frontal mechanisms are applied.
135 Although the frontal lobes appear to be less functionally committed than more posterior cortical regions,
136 their functions can be roughly divided along four spatial dimensions: left–verbal/right–nonverbal, anterior–cognitive/posterior–motor, ventral–perception/dorsal–action, and medial–internal focus/lateral–external focus. Thus, the verbal aspects of working memory tasks may activate the left dorsolateral prefrontal cortex and nonverbal aspects may activate the right.
137–139 Even within the domain of nonverbal working memory, recall of faces activates more ventral regions of the right dorsolateral frontal cortex than does recall of spatial location.
140 This functional specificity may go all the way down to the cellular level.
141 Goldman-Rakic has suggested that different prefrontal areas may perform the same operation on different inputs.
23 This hypothesis is consistent with the functional segregation of the basal ganglia–thalamocortical circuits. Support for a modular organization of frontal function has been developed in humans.
142 Cognitive test performance is most closely related to dorsofrontal cerebral glucose metabolism, whereas social behavior and disturbances of comportment are related to mesio-/orbitofrontal metabolism. Similarly, dorsal regions of the anterior cingulate are activated by attention-demanding Stroop-like interference tasks, whereas ventral regions of the anterior cingulate respond when similar tasks are applied to emotionally laden content.
143 Dimensions of Executive Control:
There are many putative ECF measures
144 (
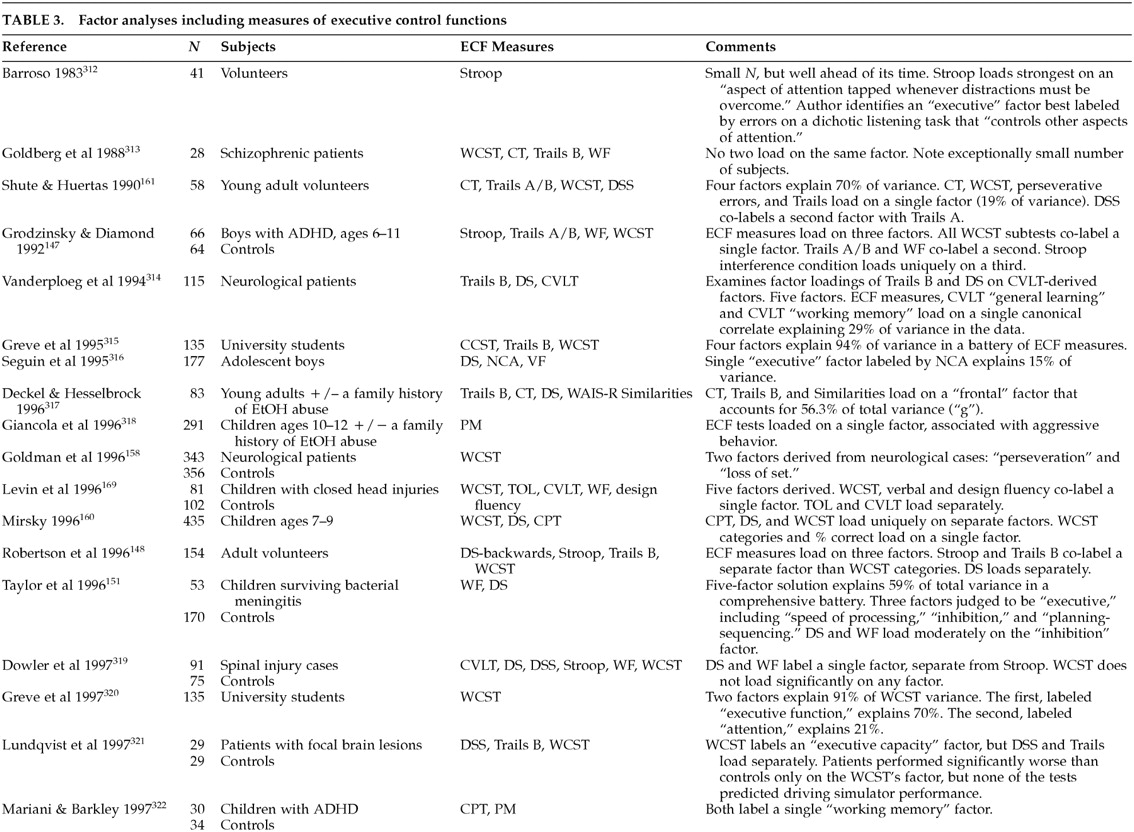
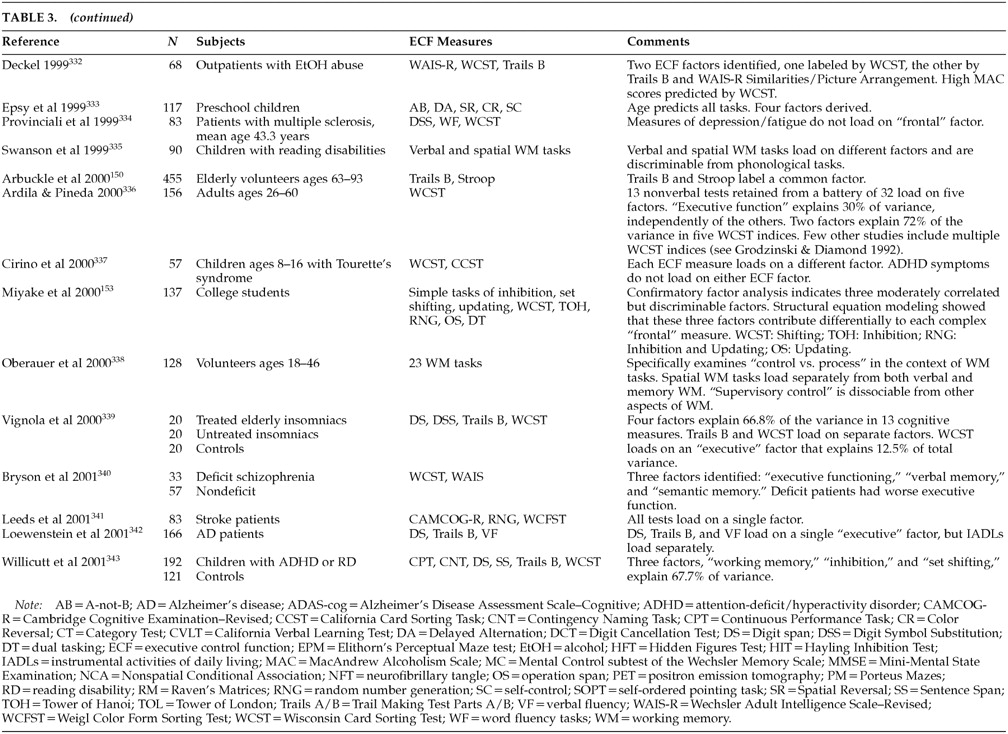
Table 2). However, it is not at all clear that these all test the same dimensions of executive control. Our literature review identified several studies containing factor analyses of putative ECF measures (
Table 3A,
Table 3B, and
Table 3C). Interpreting these studies can be difficult.
145 Few have been intentionally designed to address ECF. Prior to about 1998, most authors interpreted their results without regard to ECF or frontal function. Instead, factors with strong loadings by ECF measures were thought to represent “vigilance” or “attention.” The differences between ECF and simple attention have been extensively studied.
146 It is relevant to the cybernetic formulation of ECF that “judgment,” “concept formation,” “problem solving,” and “decision making” are seldom mentioned in factor analyses of ECF measures.
Putative ECF measures do not load onto a single, overarching executive construct. Most studies find multiple dimensions of executive control. The available studies tend to confirm a rule discovery factor labeled by tests such as the WCST categories; a working memory factor labeled by tests such as the California Verbal Learning Test, the Wechsler Intelligence Scale for Children–Revised (WISC-R), Digit Span (verbal), and the Tower of London (nonverbal); an attentional control factor labeled by tests such as the Continuous Performance Task or Digit Cancellation; and a response inhibition factor labeled by tests such as the WISC-R Digit Span Backwards, Trails B, or the Stroop. Rule discovery and working memory are most closely related to dorsolateral cortical function. Attentional control and response inhibition depend more on ventromedial regions.
These domains are fairly robust. Different authors have found the same instruments to load together in different samples. For example, Trails B and the Stroop co-label a single factor (
response inhibition) in Grodzinsky and Diamond's study of boys with ADHD,
147 Robertson et al.'s study of normal adults,
148 Mahurin et al.'s study of schizophrenic patients,
149 and Arbuckle et al.'s study of elderly adults.
150 In addition, there is limited evidence that ECF factors are multimodal. For example, Taylor et al.
151 found that both verbal and design fluency tasks loaded on the same factor. This finding suggests that the executive control identified in this paradigm may be equally applicable to both verbal and constructional processes, presumably mediated by different cerebral hemispheres.
Unfortunately, most of the available ECF factor-analytic studies have methodological flaws. Large sample sizes are needed before stable factor structures can emerge. Executive and nonexecutive measures need to be included, and key reference measures should be used across samples to facilitate comparisons.
Two recent studies can serve as models for future work.
152,153 Kanne et al.
152 examined the factor structure of a comprehensive battery of neuropsychological measures, including several ECF measures, among 407 AD patients and 261 elderly control subjects. Control data exhibited a different factor structure than that found in data for AD patients. Control test scores loaded on a single factor (i.e., they showed high “g”). In contrast, the data from AD cases was best represented by a three-factor model. The authors labeled these factors Mental Control, Memory-Verbal, and Visuospatial. Digit Span, verbal fluency, and the Mental Control subtest of the Wechsler Memory Scale loaded on the Mental Control factor. This factor explained most of the variance in both early AD and moderately advanced AD subgroups. Autopsies were later performed on 41 AD subjects. Each factor was significantly correlated with the severity of AD pathology in a different cortical region. The “Mental Control” factor correlated significantly (
r=0.39,
P=0.01) with frontal cortical neurofibrillary tangle counts. Digit Symbol Substitution, a test that is often purported to measure ECF, did not load on the Mental Control factor, nor was it correlated with frontal pathology.
Miyake et al.
153 examined putative ECF measures, including the WCST, the Tower of Hanoi (TOH), random number generation (RNG), operation span, and dual tasking in a moderately large sample of college students (
N=137). A confirmatory factor analysis of these measures indicated three moderately correlated but discriminable factors, which they labeled Set Shifting, Inhibition, and Updating. Structural equation models showed that these three factors contribute differentially to each of the “complex” ECF measures. The Set Shifting factor contributed most to WCST performance, the Inhibition factor contributed most to TOH, and both Inhibition and Updating contributed to RNG. The Updating factor also contributed to operation span scores. This type of analysis reveals that 1) classical “ECF” measures are often multidimensional; 2) no single measure comprehensively assesses all ECF domains; and 3) specific combinations of ECF measures may compliment each other, while others may be redundant.
For a discussion of the Wisconsin Card Sorting Test as a possible gold-standard ECF measure, see
box (p. 391).
FUNCTIONAL IMAGING AND EXECUTIVE CONTROL
Lesion studies associate
response inhibition with the orbitofrontal region,
attentional control with the mesiofrontal region, and
working memory (verbal and nonverbal) and
rule discovery with the dorsolateral region.
162 These observations are generally supported by neuroimaging. Bench et al.
65 studied the associations between a modified Stroop and regional cortical metabolism PET. During the Stroop's interference condition, the right orbitofrontal cortex and posterior parietal cortex were both activated (i.e., control and process). However, these regions may both be under the control of the anterior cingulate. The anterior cingulate is thought to be important in error detection and sequencing of ongoing action plans.
143 It has been shown to be activated by stimuli that are incongruent with expectation and that may need correction. Liotti et al.
163 have studied the temporal sequencing of cortical activity during the Stroop's interference condition, using event-related potentials (ERPs). Differences in ERP between Incongruent compared with Congruent trials first appear in the anterior cingulate (peaking at 410 ms), then in the temporoparietal cortex (500–800 ms post stimulus).
Working memory tasks activate dorsolateral prefrontal regions. The left hemisphere may mediate verbal working memory. The right may mediate nonverbal working memory.
138 There is some overlap between these regions and other executive tasks. Verbal fluency tests tend to activate the left dorsofrontal cortex,
164,165 although in one study a test of category fluency activated the right dorsolateral prefrontal cortex relative to a baseline reading task.
166 Tasks requiring sustained attention have also been found to activate the right dorsolateral prefrontal cortex.
167 However, the factor-analytic studies reviewed above suggest that most ECF measures are complicated tasks that may draw on several executive domains simultaneously. The Tower of London, for example, loads on two factors in Culbertson and Zillmer's study of boys with attention-deficit/hyperactivity disorder (ADHD)
168 and on three separate factors in Levin et al.'s study of head-injured children,
169 and it has been reported to activate the left dorsolateral prefrontal cortex
170 and mesiofrontal/anterior cingulate.
171 In a functional MRI (fMRI) study by Peterson et al.,
59 seven factors were derived from the brain regions activated by the Stroop. The anterior cingulate (mesiofrontal system) loaded significantly on each of these seven factors (see Liotti et al.
163 ).
The nonspecificity of putative ECF clinical measures is in sharp contrast to the relatively discrete frontal activations associated with certain tasks in neuroimaging studies. The “delayed response,” “A-not-B,” “go/no-go,” “n-back,” and “object retrieval” paradigms all reproducibly activate very specific frontal regions. However, it should be kept in mind that the skills represented by these tasks are achieved by human beings very early in development, long before clinically relevant executive skills have developed. The A-not-B, delayed response, and object retrieval paradigms are essentially in place in human infants by the age of 12 months.
172–174 Thus, these easily localized tasks, while clearly dependent on frontal functions, may be merely the heteromodal processes on which truly cybernetic “executive” functions operate.
TREATMENT OF ECF IMPAIRMENT
ECF offers a new perspective from which to study the pharmacotherapy of major neuropsychiatric disorders. Moreover, there may be regionally specific differences in ECF treatment response. Dopamine D
1 receptor agonists improve performance on working memory–related tasks that are thought to be dependent on dorsolateral prefrontal activity. The response is nonlinear (an inverted ∪ shape). Too much or too little DA activity can adversely affect function.
269 The response to DA can be predicted by performance on working memory–sensitive tasks such as Digit Span. Normal aging is associated with both diminished dopaminergic function and impaired Digit Span performance, suggesting one possible and potentially reversible explanation for age-associated cognitive decline. Norepinephrine α
2 agonists also improve working memory–related tasks,
107 whereas α
1 agonists impair working memory.
270 In contrast, serotonin deficiency impairs function on tasks that have been related to orbitofrontal activity.
271 Nevertheless, our literature review did not identify any clinical trials of ECF impairment. However, our collateral review identified many studies that could be interpreted from this perspective. Much depends on one's definition of an “executive measure.” DSS or WAIS-R subtests such as Category Formation are sometimes suggested to invoke ECF. However, they seldom co-label factors with other ECF measures. On the other hand, the recognition that frontal lesions lead to reproducible patterns of behavioral disorganization suggests that even behavioral outcomes may be sensitive to ECF-related change. We have chosen to limit our discussion to the few studies with less ambiguous executive outcomes. Initial results look promising. ECF psychometric and frontal system neuroimaging deficits have been found to respond to treatment in ADHD, major depression, and schizophrenia. Each has well-documented frontal/ECF deficits and a well-developed ECF literature.
In ADHD, studies have repeatedly demonstrated the effect of stimulants such as methylphenidate (Ritalin, Focalin),
d-amphetamine (Dexedrine) or pemoline (Cylert) on impulsive behavior and response inhibition tests.
154,272 Stimulants also appear to improve verbal and spatial working memory.
273,274 These drugs have mixed agonist effects at postsynaptic dopaminergic D
1 and noradrenergic α
2 receptors.
An emerging literature suggests that selective serotonin reuptake inhibitors (SSRIs) may have efficacy against the cognitive impairments of depression
275,276 However, all SSRIs may not be equally effective. The apathetic behavior profile of depression, along with the relationship of apathy to hypodopaminergic states, provides a rationale for the specific use of sertraline against depression-associated ECF impairment
277 (see ECF Impairment and Problem Behavior, pp. 394–395). Ventral tegmental DA inputs are directed largely toward the mesiofrontal cortex and nucleus accumbens (in the mesiofrontal circuit). Wolfe et al.
98 have associated a dysexecutive pattern of neuropsychological test scores with low cerebrospinal fluid (CSF) homovanillic acid levels in patients with Parkinson's disease, major depression, and an apathetic subset of AD cases. Similarly, negative symptoms in schizophrenia have been associated with low levels of CSF DA metabolites.
278 Sertraline, unlike the other available SSRIs, is a potent DA reuptake inhibitor.
279 It is roughly half as potent as amphetamine,
280 although this effect is not likely to be seen at usual therapeutic doses.
Keefe et al.
281 have published a meta-analysis of the effects of atypical antipsychotics on cognitive function among patients with schizophrenia. Fifteen studies were reviewed (including three double-blind controlled trials). After correction for multiple comparisons, significant improvement was found in the DSS, verbal fluency, and “executive function.” Three double-blind trials have compared the effects of atypical antipsychotics to haloperidol on cognition, using ECF measures. Schizophrenic subjects treated with risperidone have been found to perform better than those treated with haloperidol on Trails B (but not Trails A)
282 and tests of verbal working memory.
283 Schizophrenic subjects treated with clozapine have been found to perform better than those treated with haloperidol on tests of verbal fluency.
284 And schizophrenic subjects treated with quetiapine have been found to perform better than those treated with haloperidol on tests of verbal and design fluency.
285 The ability of atypical agents to improve ECF specifically would be important. Frontal metabolic function and performance on ECF measures are better indications of long-term functional outcomes than is the successful reduction of psychotic symptoms.
248 It is important to note that antipsychotic medications may have differential effects on ECF-related symptom clusters. Positive symptoms remit with traditional antipsychotic treatment, but cognitive impairment does not.
286,287 Both positive symptoms and cognition improve with the atypical antipsychotic clozapine. These differential effects may reflect frontal cortical DA receptor distributions.
288 Prefrontal cortical GABAergic interneurons (in layer IV) express dopamine D
2 289 and D
4 290 receptors that may mediate the antipsychotic effects of neuroleptics. In contrast, pyramidal cells in layers III and V express high densities of D
1 receptors. D
1 receptors mediate WCST performance in humans.
108 These receptors are downregulated in schizophrenia
291 and by conventional antipsychotic agents.
269 D
1 receptor blockade can lead to worsened performance on putative executive measures.
292–294 RESEARCH AGENDA
Much more research is needed with regard to ECF. First, there needs to be a definitive taxonomy, both of the different dimensions of executive control and of the clinical phenomena associated with them. Both questions can be approached through latent class analyses, which are useful in the absence of a gold standard.
295 This taxonomy should be independent of the features of any single disorder. “Negative symptoms,” for example, are no more specific to schizophrenia than “apathy” is to depression.
Second, neuroimaging and advanced statistical techniques are pushing us toward the limits of a localization model of executive control. Neither the executive functions themselves nor the instruments that purport to measure them map reliably into specific regions of interest. However, once factor analyses, cluster analyses, grade of membership, or discriminant modeling studies have defined the major frontal syndromes and their associated psychometric characteristics, it will be possible to map them to specific (yet distributed) neural networks. Notable advances in this regard have already taken place (e.g., Liddle and Morris's
264 approach to the neurobehavioral symptoms of schizophrenia, Mahurin and colleagues'
149 efforts to co-localize psychometric factors with distributed networks of cortical regions derived from functional neuroimaging, Kanne and coworkers'
152 pathological correlations with “frontal” factor scores, and Peterson's recent fMRI study of the Stroop
59 ).
Third, ECF needs to be incorporated into routine clinical assessment. The prevalence and severity of ECF impairment in most disorders is still unknown, but ECF impairment is likely to be common and also to predict behavioral/functional disability independently of impairment in traditional cognitive domains. Clinicians may not be appropriately trained to recognize ECF impairment at the bedside and distinguish it from affective or behavioral impairments. Similarly, cognitive assessments and screening batteries are increasingly being recognized as deficient in their ability to sensitively detect ECF impairment. This can lead to the underdetection or underestimation of cognitive impairment, particularly in those disorders that disproportionately affect frontal system function.
Fourth, the risk factors for ECF impairment need to be understood. Little is known about ECF-specific risk factors. Both genetic and environmental factors need to be considered.
Fifth, there is a pressing need for pharmacological treatment trials directed at specific ECF domains. It may be that we have already been seeing treatment-related improvement in ECF, but, without ECF-specific outcome measures, this effect is likely to be misattributed to change in “depressive symptoms,” “noncognitive behaviors,” “functional status,” nonspecific attentional factors, or other cognitive domains.