Traumatic brain injury (TBI) is an important health problem and has been widely reported to be one of the leading causes of death among young adults.
1,2 TBI affects approximately 1.5 to 2 million people in the United States each year, with an estimated 70,000 to 90,000 cases resulting in significant functional impairment.
3 In cases where TBI does not result in death, TBI survivors frequently sustain a variety of physical, neuropsychological, and emotional/behavioral impairments. Further, impairment in cognitive functioning has specifically been linked to poor long-term outcome in the areas of vocational functioning,
4–6 independent living,
7,8 and community integration.
9 Young people, particularly young men between the ages of 15 and 24, are the most common victims of TBI and are thus faced with the possibility of a lifetime of disability and handicap.
10Treatment of the cognitive and behavioral impairments associated with TBI should result in decreased handicap, improved quality of life, and decreased societal impact. Attempts at ameliorating cognitive deficits following TBI have widely focused on neurocognitive rehabilitation. Although neurocognitive rehabilitation appears to be helpful from a clinical perspective, empirical investigations to date have failed to provide conclusive evidence of efficacy in improving outcome after TBI.
3,11 Further, there are no presently established pharmacological strategies to address these outcomes in TBI. Given the lack of conclusive evidence regarding the efficacy of cognitive rehabilitation of TBI, and the lack of current pharmacological strategies to improve cognition, behavior, and quality of life in patients with TBI, investigation of new intervention strategies is clearly needed.
In this review, we present evidence for the potential benefit of cholinergic agents in treating cognitive, and possibly behavioral, impairment following TBI. We first review evidence underlying hypotheses regarding the possible efficacy of cholinergic agents in TBI and then review the literature investigating the use of cholinergic agents in TBI populations. Finally, we present recommendations regarding the types of outcome measures that should be included in clinical trials of cholinergic agents in TBI.
ACETYLCHOLINERGIC TREATMENT OF COGNITIVE IMPAIRMENT IN TBI
Methodology of the Literature Review
MEDLINE and PsycINFO listings for English-language journals were searched for the following terms: brain injury OR head injury AND cholinergic, choline, citicoline, donepezil, tacrine, physostigmine, and lecithin.
Our literature review revealed a total of 13 articles presenting original data related to the use of cholinergic agents to address cognitive impairments following TBI. These reports range from case studies to randomized, placebo-controlled designs.
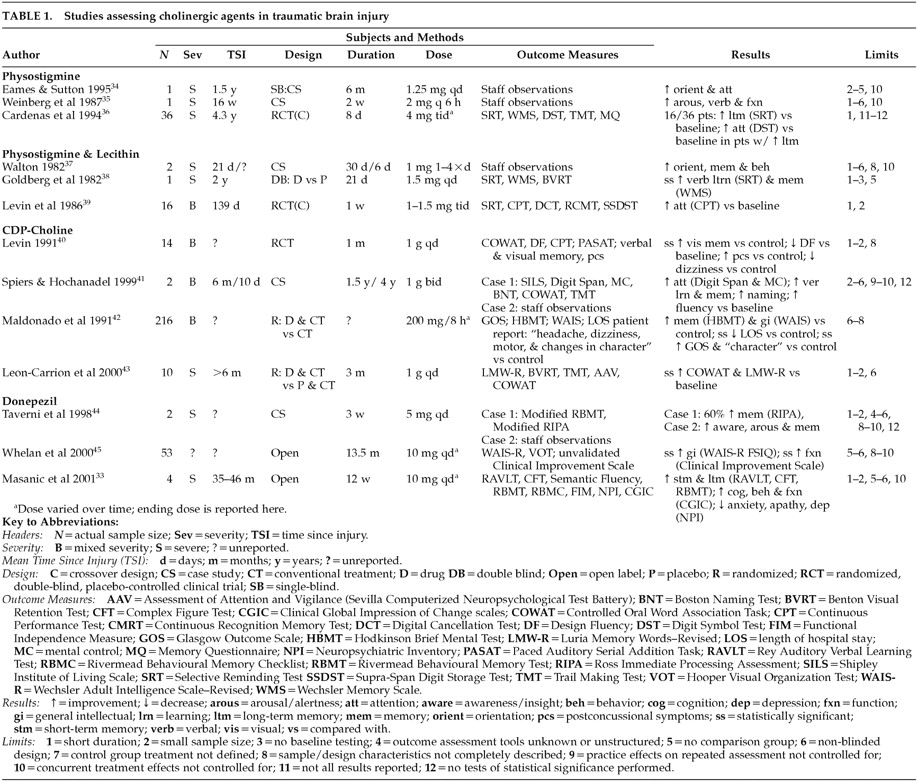
Table 1 presents a summary of these studies by drug type and highlights the limitations of each study.
Physostigmine
Two case studies have reported beneficial effects of physostigmine in patients with a history of severe TBI. Eames and Sutton
34 described a 57-year-old male with a history of TBI who was administered physostigmine intravenously on 15 occasions over the course of 2 months. No objective assessment was conducted, but the authors reported that staff who were blind to the treatment noted reduced confusion, both immediately after the injections and progressively over the course of the treatment period. Further treatment by daily injections of physostigmine for 7 consecutive days was reported to result in improved cognition and language abilities. Finally, weekly intramuscular physostigmine injections over a 6-month period were noted to result in improvements in orientation, cognition, memory, and learning, as well as a decrease in confusion and paranoid ideation.
Weinberg et al.
35 described a 33-year-old male with a history of TBI who was administered intramuscular physostigmine, combined with methylphenidate, every 6 hours for a period of 2 weeks. Although no objective assessment was conducted apart from Glasgow Coma Scale (GCS) scores, the authors reported that the patient demonstrated improvements in alertness, ability to follow commands, participation in activities of daily living (ADLs), and verbalization for up to 2 hours following each injection. The patient's GCS score was noted to increase from 12 to 14 over the course of the 2-week treatment. In both the Eames and Sutton and the Weinberg et al. cases, discontinuation of physostigmine treatment resulted in deterioration of function to pre-treatment levels. Although neither group used objective assessment techniques or controlled for spontaneous recovery, placebo effects, or the effects of concurrent treatment, these case reports present anecdotal evidence of the possible benefit of cholinergic agents in ameliorating cognitive and behavioral impairments following TBI.
Cardenas et al.
36 conducted a double-blind, placebo-controlled, crossover investigation of oral physostigmine in a group of 36 men with a history of TBI. Subjects were tested on a series of neuropsychological measures at baseline, immediately after each 8-day treatment phase, and at 1-month follow-up (see
Table 1 for measures administered). Physostigmine treatment resulted in improvements in delayed recall, storage, and retrieval of verbal information on the Selective Reminding Test (SRT) in 44% of patients; “improvement” was defined as a 50% increase on the long-term storage or the long-term retrieval scores of the SRT. In addition, in the subgroup of patients who demonstrated improved memory in response to physostigmine, improvement in divided attentional skills on the Digit Symbol Test was also noted. Results of performance on other objective measures (i.e., Wechsler Memory Scale [WMS] and Trail Making Test [TMT]) were not reported by the authors. Performance on the SRT returned to pre-drug levels on follow-up.
Overall, these preliminary findings suggest a potential beneficial effect of physostigmine in ameliorating arousal and memory disturbance in some individuals with a history of TBI. However, the short half-life of the drug combined with the apparent failure to induce sustainable cognitive improvement presents a possible disadvantage for use in long-term treatment of individuals with TBI.
Physostigmine and Lecithin
The utility of physostigmine combined with lecithin, a choline precursor, has also been investigated in a variety of reports. Walton
37 described two clinical cases in which intramuscular physostigmine injections were combined with lecithin to treat postconcussive symptoms following TBI. No objective outcome assessment was conducted with either patient. A 34-year old female patient with a history of severe TBI was noted to show clinical improvements 48 hours after commencement of treatment. Specifically, improvements in orientation, short-term memory, and decreased aggression were noted and were reported to progress over the next 14 days of treatment. This patient's functioning returned to pre-treatment levels once physostigmine and lecithin were stopped. A 23-year-old male patient who began treatment with physostigmine and lecithin four times daily, immediately following admission to the hospital with symptoms of confusion and confabulation, was noted to demonstrate improvements in behavior and orientation within 72 hours of initiation of treatment. No follow-up information was presented on this patient. Again, these case reports did not provide objective assessment results and did not control for spontaneous recovery, placebo effects, or the effects of concurrent treatment; however, they provide additional anecdotal evidence of the potential role of cholinergic agents in treating sequelae of TBI.
Goldberg et al.
38 presented the results of a double-blind, placebo crossover investigation of oral physostigmine and lecithin in a 36-year-old man with a history of severe TBI. The patient was presented alternately with treatment or placebo three times daily over four 3-day periods, with neuropsychological assessment following each treatment/placebo administration (see
Table 1 for measures administered). Statistically significant improvements in overall memory performance on the Wechsler Memory Scale and verbal memory storage and retrieval on the SRT were noted during the treatment phase, when compared with the placebo phase. No follow-up assessment was performed to assess the long-term effects of treatment.
Levin et al.
39 conducted a double-blind, placebo-controlled, crossover investigation of physostigmine and lecithin in 16 male patients with a history of moderate to severe TBI. Subjects were assessed at baseline, after 1 week of treatment with lecithin and oral physostigmine or placebo, and after a 1-week washout period (see
Table 1 for measures administered). Results indicated that patients who were treated with physostigmine first demonstrated improvement in sustained attention on the Continuous Performance Test (CPT), when compared with patients treated with placebo first; this improvement was maintained during the washout phase.
Taken together, these findings present further evidence for a possible beneficial effect of acetylcholinesterase inhibitors, combined with choline precursors, in ameliorating cognition and behavior in some individuals with a history of TBI. Again, the improvement appears to be transitory.
CDP-Choline
Cytidine diphosphoryl choline (CDP-choline or citicoline), a choline precursor, has been reported to ameliorate certain sequelae of TBI in several studies. Levin
40 conducted a randomized, double-blind, placebo-controlled study of CDP-choline in 14 patients with a history of TBI. After emerging from posttraumatic amnesia, patients were administered either oral CDP-choline or placebo for 1 month. Neuropsychological assessment was conducted at baseline and following 1 month of treatment (see
Table 1 for measures administered). Patients were also administered a structured interview related to postconcussional symptoms. Results indicated that, compared with placebo, patients treated with CDP-choline experienced greater improvement in visual recognition memory from baseline levels. Further, compared with placebo, patients in the CDP-choline group reported fewer postconcussional symptoms after treatment, including less headache, dizziness, and tinnitus. No follow-up assessment was conducted to ascertain whether the improvement in visual memory or postconcussional symptoms was sustained following termination of treatment.
Spiers and Hochanadel
41 described two cases in which CDP-choline treatment appeared to facilitate cognitive improvement following TBI. A 39-year-old female was treated with CDP-choline for 1.5 years following a moderate TBI. Testing was performed at 6 months and 2 years after injury (see
Table 1 for measures administered). The patient was noted to demonstrate improvement on tasks of verbal attentional span, mental control, word fluency, and confrontation naming. While no baseline testing of verbal or visual memory was conducted, the patient's 2-year follow-up performance fell in the superior range on the California Verbal Learning Test (CVLT), Complex Figure Test (CFT), and Auditory Consonant Trigrams (ACT). A 41-year-old male was also treated with CDP-choline for 4 years following a severe TBI. Although no neuropsychological assessment was conducted, the patient reported improvement in arousal and alertness following initiation of treatment at 10 days postinjury. These reports, although anecdotal and uncontrolled, provide preliminary evidence for the potential efficacy of choline precursors in improving cognition following TBI.
Maldonado et al.
42 performed a single-blind randomized study of CDP-choline in 216 patients with moderate to severe TBI. Patients were randomly assigned to receive either conventional treatment (not described by the authors) or CDP-choline in addition to conventional treatment. Length of treatment was variable, depending on the progression of the patient's symptoms. Outcome was assessed with the Glasgow Outcome Scale (GOS), administered 3 months after injury. In addition, assessment of self-report of symptoms (e.g., headache, dizziness, motor dysfunction, and changes in character), Hodkinson Brief Mental Test (HBMT) of memory, and the Wechsler Adult Intelligence Scale (WAIS) was conducted when the patient left the intensive care unit and again at 3 months after injury. Results revealed that improvement in “changes in character” (not described by the authors) from baseline was statistically greater in the CDP-choline group; in addition, there was a trend toward improvement in motor symptoms (again not described by the authors) and WAIS performance in the CDP-choline group. Further, the percentage of patients falling in the “good recovery” range on the GOS after treatment was statistically greater in the CDP-choline group.
Leon-Carrion et al.
43 also reported on the use of CDP-choline in 10 patients with a history of severe TBI. Patients were randomly assigned to receive either CDP-choline combined with memory rehabilitation or placebo combined with memory rehabilitation. Neuropsychological testing was conducted at baseline and at the end of the 3-month treatment period (see
Table 1 for measures administered). Results indicated that patients in the CDP-choline group demonstrated statistically significant improvement in verbal memory (Luria Memory Words test) and verbal fluency (Controlled Oral Word Association Task [COWAT]), when compared with baseline; these improvements were not observed in the placebo group. The authors concluded that CDP-choline facilitates neuropsychological rehabilitation in patients with a history of TBI.
Donepezil
More recently, donepezil has become a drug of interest in TBI, because of its longer-acting properties and oral administration, when compared with physostigmine, and its recent approval for the treatment of AD in the United States and Canada. Taverni et al.
44 described the use of donepezil in 2 patients with a history of severe TBI. A 21-year-old female with a history of TBI was treated with donepezil daily for 3 weeks; assessment of cognition was conducted at baseline and after 3 weeks, using an unvalidated test that incorporated modified versions of the Rivermead Behavioural Memory Test (RBMT) and the Ross Immediate Processing Assessment (RIPA). Results indicated 60% improvement from baseline. Further, subjective reports by staff and family indicated improvements in awareness, adaptive functioning, recall, and participation in group discussions. A 46-year-old male with a history of TBI was treated with donepezil daily for approximately 3 weeks. No objective measures were administered, but staff noted improvement in alertness and recall. The authors did not provide follow-up information regarding whether improvements were sustained following discontinuation of treatment.
In a recent open-label trial, Whelan et al.
45 administered donepezil to 53 patients with a history of TBI for up to 2 years. Assessment was conducted at baseline and, on average, 12 months following initiation of treatment (see
Table 1 for measures administered; no memory measures were used in this study). In addition, the first and second authors completed a “Clinician Improvement Scale,” which rated subjects according to improvement of function (e.g., mood, affect, energy, interest in daily activities, grooming, and social interaction) from baseline. Results indicated statistically significant improvement in WAIS-R Full Scale IQ scores, when compared with baseline, as well as statistically significant improvement in clinician ratings of function, when compared with baseline.
Our group recently completed an open-label trial of donepezil in 4 patients with TBI.
33 We found that a 12-week trial of donepezil resulted in statistically significant improvements to 0.4, 1.04, and 0.83 standard deviations above baseline values on the RAVLT total recall, short-term recall, and long-term recall scores, respectively. Performance also improved to 1.56 and 1.38 standard deviations above baseline values on the CFT short-term and long-term recall scores, respectively. Although no improvement in functional abilities, as assessed by the Functional Independence Measures (FIM) and the Clinical Global Impression of Change (CGIC) scale, was associated with improved cognition, we hypothesized that a longer medication trial might be required in order to observe functional improvements in these patients. Our results also indicated improvements in emotional/behavioral functioning in patients treated with donepezil. Specifically, decline in anxiety, depression, and apathy scores on the Neuropsychiatric Inventory (NPI)
46 was noted. This finding highlights the possibility of treating behavioral, in addition to cognitive, sequelae of TBI with acetylcholinesterase inhibitors.
ACETYLCHOLINERGIC TREATMENT OF APATHY IN TBI
Personality changes, including irritability, impulsivity, emotional lability, aggression, and apathy are commonplace following TBI. Apathy is characterized by diminished initiative, diminished interest and concern, and diminished emotional responsiveness.
47 Apathy following TBI is particularly problematic, as it can often have a negative impact on rehabilitation, return to work, and successful reintegration to the community following TBI.
48 Estimates of the frequency of apathy following TBI range from 46%
49 to 71%.
48There is growing evidence for a link between cholinergic dysfunction and the behavioral syndrome of apathy. Limbic and paralimbic structures are among the regions containing the highest acetylcholine levels in the brain.
50 It is hypothesized that the nucleus basalis, which manufactures the choline acetyltransferase necessary for synthesis of acetylcholine and serves as a structural link between the limbic system and the cortex, may contribute to symptoms of apathy.
51 Specifically, the nucleus basalis is believed to exert influence on the cortex in response to motivational and emotional information conveyed through limbic structures,
52 and dysfunction of this structure may contribute to decreased ability to link emotional significance to other information about the world.
The relationship between apathy and the cholinergic system has been most extensively studied in Alzheimer's disease. Apathy is one of the most commonly reported behavioral symptoms of AD, estimated to occur in 72% of AD patients.
51 There is growing evidence that cholinergic agents result in improvement in behavioral symptoms of AD, including apathy.
53,54 Specifically, tacrine and metrifonate (another acetylcholinesterase inhibitor) have been used with AD patients to produce statistically significant declines in apathy.
30,55 In a review of studies of cholinergic treatment of behavioral symptoms in AD, Cummings
30 concluded that, along with visual hallucinations, apathy is the most consistent behavioral symptom to improve. Cummings hypothesized that the strong responsiveness of apathy to cholinergic agents may be related to improvement in attentional systems associated with cholinergic treatment; Cummings also suggests that the behavioral deficits common to other disorders associated with cholinergic dysfunction, such as TBI, may be treatable with cholinesterase inhibitors, as well.
Although cholinergic mechanisms of behavioral disorders in TBI have received relatively little attention, extrapolation from AD research suggests a rationale for treating the syndrome of apathy with cholinergic agents. Indeed, in an uncontrolled trial of 4 patients with a history of TBI, our group found that donepezil had a beneficial effect on apathy, as measured by the NPI.
33 The present literature review revealed no additional studies addressing treatment of apathy with cholinergic agents following TBI. However, the prevalence of apathy following TBI suggests a need for further investigation of treatment mechanisms and strategies. Further investigation of the possible role of cholinergic agents in ameliorating other behavioral deficits common to both AD and TBI (e.g., irritability, disinhibition) is also needed.
OUTCOME MEASURES IN FUTURE STUDIES OF CHOLINERGIC AGENTS IN TBI
We propose that the following criteria be considered when selecting outcome measures for inclusion in future studies of cholinergic agents in TBI: 1) whether the measures address the spectrum of cognitive and/or behavioral realms commonly affected in TBI; 2) whether specific measures have demonstrated responsivity to acetylcholinergic manipulation in prior studies; and 3) whether the measures have demonstrated positive relationships with broader functional outcome in TBI.
The selection of appropriate outcome measures should also be guided by our understanding of the role of the cholinergic system in human cognition. It has been hypothesized that the cholinergic system serves to direct attentional processes to relevant stimuli, and that cholinergic disruption diminishes this ability.
12 Other authors
30 have suggested that the primary effect of cholinergic agents on cognition may be through their action on the attentional/executive system, which may have a modulating influence on other cognitive skills, including memory. One might postulate that the effect of cholinergic agents on cognition following TBI may be to improve the executive/control system, thereby resulting in improvements in performance on aspects of all cognitive processes (attention, memory, language, etc.) at lower levels.
These hypotheses lead us to suggest the need for a broader range of outcome measures in studies assessing the role of cholinergic agents in TBI. Few studies to date have included objective assessment of skills other than memory and attention. We hypothesize that cholinergic agents, through effects on basic attentional/executive systems, might be demonstrated to influence cognitive function in a variety of realms, if studied appropriately. In particular, we recommend that future studies include outcome measures that assess a broad range of cognitive abilities typically affected in TBI, including verbal and visual memory (e.g., RAVLT,
56 CFT
57), working memory (e.g., Brown-Peterson task
58), sustained, selective and divided attention (e.g., CPT,
59 Digit Symbol Test,
60 TMT
61), and executive functioning (e.g., Wisconsin Card Sorting Test,
62 COWAT
63). Further, more extensive investigation of cholinergic agents in the treatment of behavioral deficits following TBI is also indicated by the above review. We expect that cholinergic agents will improve symptoms of apathy in TBI, and recommend that future studies include instruments that address these realms (e.g., Apathy Evaluation Scale–Informant and Self-rated versions
47). Finally, although speculative at this point, one might hypothesize that behavior in other realms (e.g., irritability, disinhibition) may also be improved through use of cholinergic agents. These realms should be addressed through use of a broader array of outcome measures designed to assess behavior following TBI, including the Katz Adjustment Scale,
64 the Neurobehavioral Rating Scale,
65 and the Portland Adaptability Inventory.
66Inclusion of measures that have demonstrated responsivity to acetylcholinergic manipulation in prior studies of normal controls, AD patients, and/or TBI patients might also prove useful in providing us with a greater understanding of the relationship between cognition and the cholinergic system. In particular, measures of verbal learning, including the RAVLT and the SRT, have been consistently demonstrated to respond to cholinergic agents in studies of both TBI
33 and AD populations.
17Finally, we recommend that measures which have demonstrated relationships with broader TBI outcome be included in future studies. For example, performance on specific measures of verbal memory (i.e., RAVLT; CVLT; WMS Logical Memory subtest), visual memory (WMS), and attention/executive functioning (TMT, WCST) has been found to be correlated with return to work or school,
4,6,67 general outcome,
68 and community integration
9 following TBI. Further, we recommend that measures which directly assess quality of life (e.g., Sickness Impact Profile
69), functional performance (e.g., Patient Competency Rating Scale
70), and community integration (e.g., Community Integration Questionnaire
71) be included in future studies. Clearly, improvement in performance on discrete tasks of memory and attention is encouraging; however, our goal should be to demonstrate that such improvements translate into sustainable changes in functional abilities and quality of life for patients who are being treated.
CONCLUSIONS
Although many of the studies presented above are anecdotal case reports, and thus fail to control for the effects of spontaneous recovery, placebo effects, practice effects, and the effects of concurrent treatment, they do provide preliminary evidence to support further investigation of cholinergic agents in treating cognitive and behavioral impairments following TBI. Further, several small controlled trials have provided evidence for statistically and clinically significant efficacy of these agents in ameliorating specific cognitive deficits following TBI. The accumulating data do provide solid preliminary evidence that cholinergic agents are likely to be efficacious in ameliorating attention, memory, and perhaps executive impairment in some patients who have sustained a TBI. The apathy syndrome, and perhaps other aspects of behavior, may also improve. These more molecular improvements are likely to result in improved outcome and quality of life for individuals with TBI. However, there is a strong need for large-sample, randomized, double-blind, placebo-controlled studies of specific cholinergic agents in TBI populations. Such studies should include a broad range of outcome measures as suggested herein.