The use of ketamine instead of methohexital anesthesia has been recommended as a possible means to potentiate the effectiveness of ECT because it appears to have fewer anticonvulsant effects.
1,2 This would be expected to be particularly important for those patients whose seizure thresholds, at some point during the ECT course, approach or even exceed the maximum stimulus intensity presently allowed in U.S. ECT machines by the Food and Drug Administration.
3,4 In this regard, we have recently found that for the 5% of patients in whom the maximum available stimulus intensity elicits seizures lasting less than 25 seconds (referred to hereafter as “short” seizures), the therapeutic response rate was only 32%, compared with 63% for all other patients.
4 Ketamine might also be expected to be useful for increasing treatment effectiveness for many patients receiving unilateral ECT, in which efficacy appears to increase at greater stimulus intensities above the seizure threshold.
5,6Thus far, there have been two single reported cases where ketamine anesthesia appeared to be associated with longer seizure duration than methohexital.
7,8 A subsequent report of 10 patients switched from methohexital anesthesia to ketamine following short seizures failed to find such an effect.
2Our clinical experience has been that many patients who have short or absent seizures at the maximum available stimulus intensity with the use of methohexital anesthesia have seizures lasting longer than 25 seconds when switched to ketamine anesthesia. As a result, we carried out a retrospective analysis of data on 36 patients who were switched from methohexital to ketamine anesthesia, primarily because of short seizures at the maximum stimulus intensity. This within-subjects comparison of methohexital and ketamine did not allow a direct assessment of the relative efficacy or effects on seizure threshold of these two agents. Instead, as a step toward determining whether there is clinical utility in making the switch to ketamine when short seizures are elicited with methohexital at the maximum available stimulus intensity, we sought to determine whether this switch addressed the abbreviated seizure duration, which has been associated with diminished therapeutic response.
4We also studied whether the switch to ketamine led to ictal EEG evidence of increased seizure intensity as a possible indicator of a decrease in seizure threshold and enhancement of efficacy. Our interest in this question was based on prior evidence that one measure of greater seizure intensity (greater slow-wave amplitude) has been reported to be increased with ketamine as compared with methohexital anesthesia and that ictal EEG evidence of greater seizure intensity has been associated with a greater therapeutic response and with stimuli that exceed the seizure threshold to a greater extent.
8,9In addition, we sought to assess the safety of ketamine anesthesia with ECT. Concerns about safety have limited the use of this anesthetic agent because of evidence that it can be associated with transient increases in blood pressure and pulse, ataxia, nausea, agitation, hallucinations, and flashbacks, although the psychological effects have not been reported with ECT.
2,7,8,10,11Lastly, we sought to determine whether ketamine was associated with less ECT-associated cognitive impairment than methohexital anesthesia. One study examined the differential effects of these agents on post-treatment reorientation time, which appears to be statistically related to the degree of retrograde amnesia with ECT.
10,12 A longer reorientation time (by 5.6 minutes), suggesting greater retrograde amnesia, was reported with ketamine. However, the authors did not report subjects' age, the point in the treatment course at which ketamine or methohexital was given to the subjects, and whether the comparison was made between subjects or within an individual over the treatment course.
10 Because of the prime concern of memory side effects with ECT and preclinical evidence for a memory-sparing effect of ketamine in the context of electrically induced seizures,
13–18 we carried out a reexamination of the relative effects of methohexital and ketamine on post-treatment reorientation time.
METHODS
Subjects
Subjects were 36 patients who received ketamine anesthesia in a retrospective review of all 723 patients who received index ECT treatment at Duke University Medical Center from 1989 through 1998 following informed consent. Six subjects who received ketamine anesthesia were not included in the study because they received a mixture of ketamine and low-dose methohexital and there is evidence that in the low-dose range methohexital may have proconvulsant properties.
19,20 One subject who received ketamine had received it at every treatment and thus could not be included. Of the 36 subjects, 17 were men and 19 were women, and the mean age was 67.6 years (SD=16.0). Nineteen had unipolar major depression, 15 had bipolar depression, and 2 had schizoaffective disorder, depressed (DSM-III-R).
Post-treatment reorientation time was studied as a measure of cognitive side effects.
12 These assessments were performed on all of the subjects; however, we were able to obtain complete reorientation time data from the medical records for only 14 of the subjects. All of these 14 subjects had no evidence of neurologic disease on the basis of history and neurologic exam.
ECT Administration
All subjects received ECT
4 administered by using either a MECTA-SR1 or SPECTRUM 5000Q (MECTA Corp., Lake Oswego, OR) or a Thymatron-DGx (Somatics Corp, Lake Bluff, IL) ECT device. Ketamine and methohexital treatments that were compared were always administered with the same ECT device. Seizure threshold was titrated at treatment 1,
21 and subsequent treatments were administered at 2.25 times the seizure threshold for right unilateral (RUL) ECT and 1.5 times threshold for bilateral (BL) ECT. Of the 36 subjects, 22 received BL ECT and 14 received RUL ECT. Seizures that were less than 25 seconds in duration as determined with the EEG were followed by restimulation at the same treatment session at a 50% increase in stimulus intensity unless this occurred at the maximum stimulus charge available (576 mC MECTA, 504 mC Thymatron-DGx).
22 Routine anesthetic agents included 100% oxygen by mask, and initial dosages of succinylcholine 1 mg/kg iv, methohexital 1 mg/kg iv, adjusted as needed over the treatment course. Ketamine dosage was determined on an individual basis by the treating anesthesiologist and ECT psychiatrist. The ketamine dosing ranged from 0.7 to 2.8 mg/kg, with an average dose of 1.31 mg/kg (SD=0.31). Ketamine was administered as a single intravenous bolus of a 50 mg/ml solution. Blood pressure and pulse were assessed prior to treatment and 1 minute, 3 minutes, and 5 minutes following the ECT stimulus and periodically thereafter.
Indications for Switch to Ketamine Anesthesia
The switch to ketamine took place because a seizure of shorter than 25 seconds' duration was produced at the maximum available stimulus intensity in 24/36 subjects. For 10/36, the switch occurred because of sequential decreases in seizure duration rapidly approaching 25 seconds. In addition, one subject was switched to ketamine because of hypotension with methohexital (which resolved with ketamine), and the final subject was switched to ketamine because of methohexital-induced pruritus. The average treatment number at which ketamine was first administered was 8.7 (SD=5.1).
The comparisons between methohexital and ketamine were carried out with data from consecutive index ECT treatments that occurred immediately before and after the switch from methohexital to ketamine.
EEG Recording and Computer EEG Analysis
Two channels of EEG data were recorded from left and right prefrontal to ipsilateral mastoid Ag/AgCl electrodes.
23 The EEG data were digitized at 256 Hz with 12-bit accuracy, using a microcomputer-based EEG acquisition and analysis system (EEGSYS, Friends of Medical Science, Inc., Bethesda, MD).
Manual rejection of EEG artifacts was performed by A.D.K. blind to anesthetic agent. Fast Fourier transformation was employed to split the EEG data into three frequency bands (2–5 Hz, 5.5–13 Hz, and 13.5–30 Hz), and spectral amplitude and interhemispheric coherence (the correlation between the hemispheres within a frequency band) were computed for the immediate poststimulus, midictal, and immediate postictal periods.
23 The elapsed time before 2–5 Hz spectral amplitude exceeded the amplitude of the other two frequency bands (referred to as time until slow-wave onset) was also calculated.
23Cognitive Assessment
Cognition was assessed by measuring post-treatment reorientation time.
12 Questions regarding name, age, date of birth, location, and day of the week were administered by the nursing staff once subjects were moved into the recovery room and were repeated every 15 minutes until the subjects either got all five answers correct or 120 minutes had elapsed (in which case a score of 120 minutes was assigned). This assessment was carried out blind to the type of anesthesia administered and to the study hypotheses.
Statistical Analysis
All analyses were carried out by using the SAS system (SAS Institute, Cary, NC) and involved two-tailed tests of significance.
RESULTS
Seizure Duration
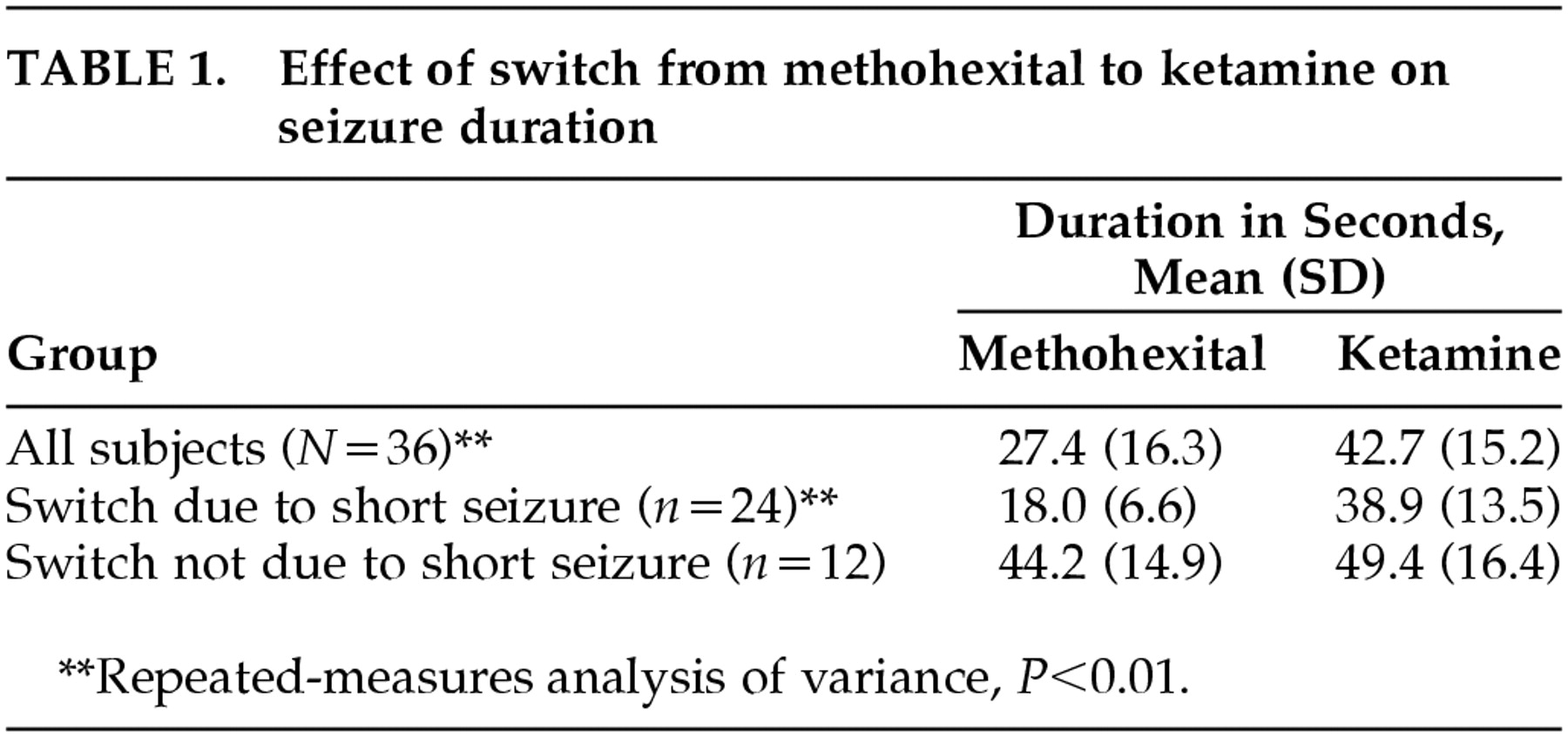
Seizure duration was prolonged with ketamine in 30/36 subjects (83%) (
Table 1). For 23/24 subjects who had shorter than 25-second seizures with methohexital, a switch to ketamine anesthesia was associated with prolongation of the seizure duration to greater than 25 seconds. In one case, it was not possible to elicit a seizure with the maximum available stimulus intensity with methohexital, and the switch to ketamine resulted in a 69-second seizure at the subsequent treatment. Repeated-measures analysis of variance revealed a significant effect of the switch to ketamine (
F=21.9, df=1 , 34,
P<0.0001) and a significant interaction between the type of anesthesia used and whether or not the switch occurred because of a short seizure with methohexital (
F=7.9, df=1 , 34,
P=0.009). Follow-up analysis indicated that seizure duration was significantly longer with ketamine than with methohexital overall (increased from 27.4 to 42.7 seconds) and was particularly prolonged when the switch to ketamine occurred because of a shorter than 25-second seizure with methohexital (increased from 18.0 to 38.9 seconds;
Table 1). There were no significant effects or interactions with electrode placement, age, or ketamine dosage.
Ictal EEG
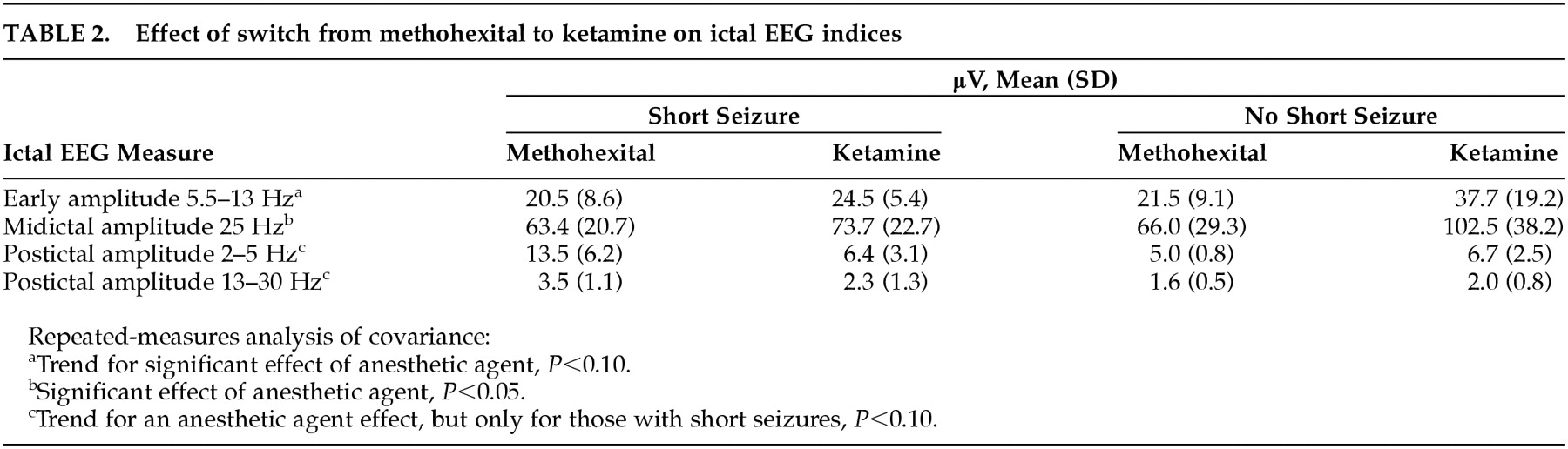
Repeated-measures analysis of covariance indicated no significant main effect for type of anesthesia alone, nor was ketamine dosage a significant covariate, but there was a significant interaction between anesthetic and whether there was a short seizure with methohexital (
F=7.2, df=1 , 5,
P<0.05), reflecting ictal EEG evidence of greater seizure intensity when the switch to ketamine followed a short seizure with methohexital (
Table 2). There was a trend for lower postictal amplitude (greater postictal suppression) following the switch to ketamine, but only for those who had short seizures with methohexital (
F=5.0, df=1 , 5,
P<0.10). For ictal amplitude, there was a significant increase in midictal low-frequency EEG amplitude (
F=8.2, df=1 , 5,
P=0.04) and a trend for greater immediate poststimulus 5.5- to 13-Hz amplitude (
F=4.4, df=1 , 5,
P=0.09) associated with the switch to ketamine without a significant interaction with whether the switch occurred because of a short seizure.
Post-Treatment Reorientation Time
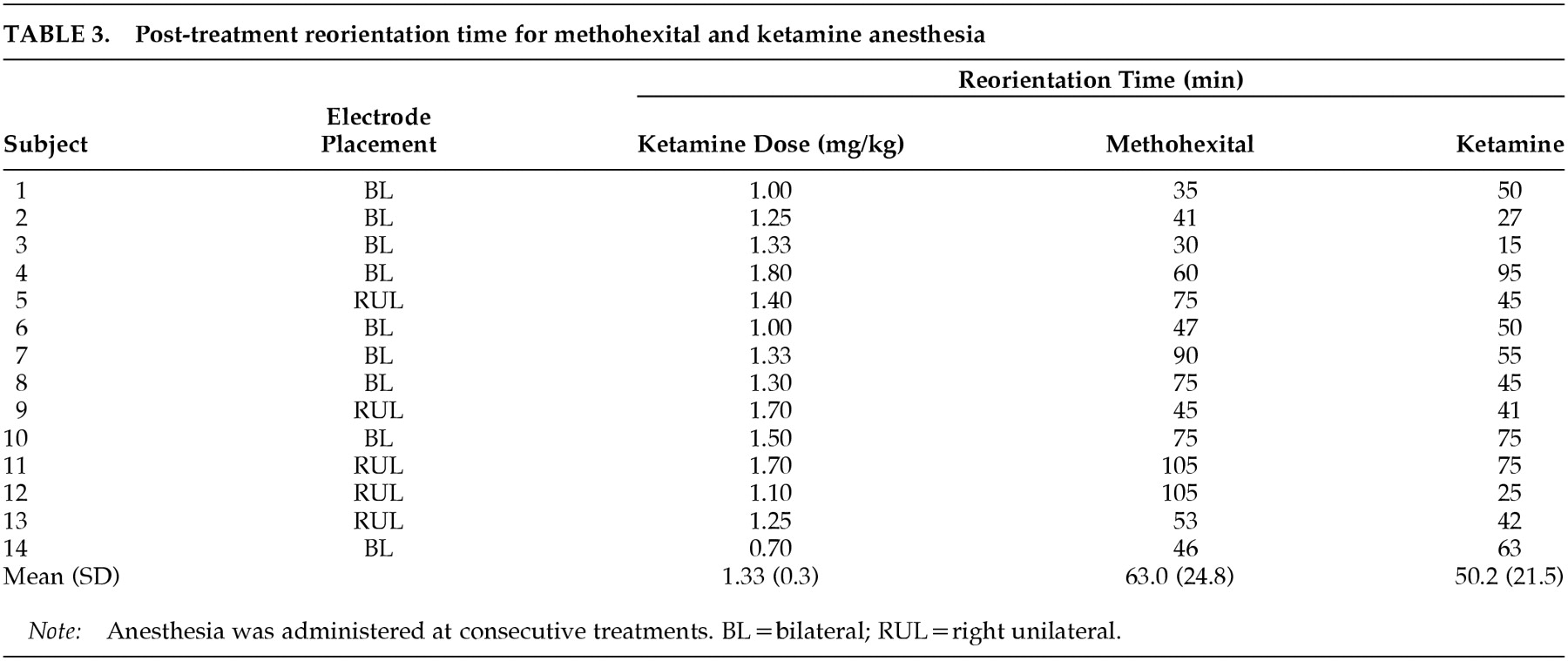
Repeated-measures analysis of covariance with reorientation time data revealed a significant main effect for anesthetic agent (
F=5.6, df=1 , 12,
P=0.04), which reflected a shorter reorientation time with ketamine (50.2 min) versus methohexital (63.0 min) (
Table 3). There was also a trend for an interaction between anesthetic agent and electrode placement (
F=3.9, df=1 , 12,
P=0.07), reflecting the tendency for those who received RUL ECT to have a greater decrease in reorientation time with the switch to ketamine. There were no significant effects of age, ketamine dosage, or whether the switch to ketamine occurred because of a short seizure with methohexital.
Side Effects of Ketamine Anesthesia
No patient experienced any adverse medical outcomes with ketamine anesthesia. Blood pressure and pulse data were available for all assessment time points for both the methohexital and ketamine treatments in only 12 subjects. Repeated-measures analysis of variance indicated that peak pulse rate and peak systolic pressure did not differ as a function of anesthetic agent; however, there was a trend (F=3.6 P<0.10) toward greater peak diastolic blood pressure with ketamine (mean±SD, for methohexital: peak blood pressure=188.9±22.1/100.1± 15.0, peak pulse=94.2±11.8; for ketamine: peak blood pressure=199.4±27.2/110.0±19.7, peak pulse=96.2± 18.0). Only 3 of the 36 subjects who received ketamine experienced any side effects. Two of these subjects experienced new onset of agitation while emerging from anesthesia. In both cases, this was alleviated at successive treatments with the intravenous administration of 1 mg of midazolam immediately following the ECT seizure. One 56-year-old subject, in whom no seizure could be elicited with the use of methohexital at the maximum available stimulus intensity, experienced hallucinations with ketamine lasting the entire day post-ECT. These hallucinations were not alleviated by the administration of intravenous haloperidol and recurred with repeated administrations of ketamine. As a result, ECT had to be discontinued. It is noteworthy that this subject was the only one with a significant history of alcohol dependence and also received the highest dose of ketamine (2.8 mg/kg). This subject was not included in the reorientation time analysis because we were not able to obtain his reorientation data from the medical records. Data from this subject do not appear in Table 3.
DISCUSSION
Effects of a Switch to Ketamine on Seizure Duration
Our results indicate that a switch to ketamine increases ECT seizure duration, and that this effect is greatest when the duration at the preceding methohexital treatment was less than 25 seconds. Greater seizure duration with ketamine is particularly notable because ketamine was always administered later in the treatment course than methohexital, and thus shorter seizures would have been expected as an order effect.
2,24The finding of seizure prolongation with a switch to ketamine agrees with the report of Lunn et al.
7 but differs from that of Rasmussen et al.
2 for unclear reasons. The latter group appears to have utilized a relatively high stimulus dose. This was a potential confound because the relationship between stimulus intensity and seizure is curvilinear, such that, if the stimulus intensity is relatively high, a decrease in seizure threshold achieved by a switch to ketamine might be expected to actually decrease the seizure duration.
24 In contrast, when the stimulus intensity is relatively close to the seizure threshold, the available evidence suggests that decreasing the seizure threshold with a switch to ketamine could be expected to increase the seizure duration.
24 Also, in the study by Rasmussen et al., methohexital and ketamine treatments sometimes differed in the use of caffeine,
2 which itself is known to lengthen seizures.
25It must be considered whether there is clinical value in prolonging the seizure duration from less than 25 seconds to greater than 25 seconds. Available evidence suggests that eliciting a seizure exceeding 25 seconds is not sufficient to achieve therapeutic adequacy;
24 however, it remains to be determined whether seizures that are shorter than 25 seconds in duration are of diminished effectiveness. Our prior study suggests that in the particular circumstance studied herein, where seizures shorter than 25 seconds occur over the treatment course at the maximum available stimulus intensity, there is a significant decrease in therapeutic response.
4 Although we have shown that a switch to ketamine leads to seizures longer than 25 seconds in this circumstance in many individuals, it remains unknown whether this intervention actually improves efficacy. Because it was not possible for us to directly compare treatment efficacy with ketamine and methohexital anesthesia because of the within-subjects comparison carried out, further studies are needed to determine if prolonging seizure duration by switching to ketamine anesthesia improves therapeutic response.
In addition, ketamine might enhance therapeutic effectiveness by lowering seizure threshold compared with methohexital anesthesia, which would be expected to lead to increased efficacy, particularly with unilateral ECT.
5,6,24 Although no data were collected in this study that directly address this issue, the observation that in one subject it was not possible to elicit a seizure with methohexital at the maximum available stimulus intensity but the switch to ketamine resulted in a 69-second seizure provides a preliminary suggestion that ketamine may raise the seizure threshold less than methohexital in some individuals.
1,7,8 Further studies are needed to determine if this is the case. In addition, it will be useful to study the relative utility of a switch to ketamine versus the implementation of other means that have been reported to potentiate seizure activity when it is difficult to elicit a vigorous seizure at the maximum available stimulus intensity, such as caffeine,
25 hyperventilation,
26 and the use of particularly long stimulus duration
27 or short stimulus pulse width.
28Ictal EEG Effects of a Switch to Ketamine
We found ictal EEG evidence of greater seizure intensity with a switch from methohexital to ketamine anesthesia. Significantly greater midictal amplitude accompanied the use of ketamine anesthesia. There was a trend for greater postictal suppression with ketamine, but only when the switch followed a less than 25-second seizure with methohexital. As with the seizure duration analyses, the effect of a switch to ketamine may have been underestimated because of the order of administration of ketamine and methohexital.
29Although postictal suppression has not been compared for methohexital and ketamine anesthesia, greater ictal amplitude with ketamine compared with methohexital has been previously reported.
8,9 Both greater ictal amplitude and postictal suppression with ketamine anesthesia are consistent with the induction of a more intense seizure with ketamine and not solely a seizure prolongation effect and are compatible with a lesser anticonvulsant effect of ketamine compared with methohexital anesthesia. Evidence that greater ictal amplitude and postictal suppression are associated with a better therapeutic response
23,30–34 also may suggest that ketamine augments therapeutic effectiveness in the context of ineffective treatments with methohexital anesthesia. It should be noted, however, that the relationship between greater ictal EEG amplitude and postictal suppression and therapeutic response has been observed only with the use of methohexital anesthesia and may not exist when ketamine is used.
23,30–34 Given this consideration and that it was not possible to directly compare the relative treatment efficacy associated with methohexital and ketamine anesthesia in the present study, further studies are needed to determine whether ictal EEG evidence of enhanced seizure intensity with a switch to ketamine is associated with enhancement of therapeutic effectiveness.
Cognitive Effects of Ketamine vs. Methohexital
The finding of shorter post-treatment reorientation time following a switch to ketamine provides very preliminary evidence that less ECT-associated retrograde amnesia may be expected with ketamine than methohexital anesthesia.
12 It also contradicts a previous study where longer reorientation time was observed with ketamine.
10 As noted earlier, that study does not report enough methodologic detail to assess the role of potentially significant confounds. In addition, our study may have underestimated the effect of ketamine due to the order of administration,
35 although greater cognitive dysfunction later in the treatment course has been disputed.
36 Shorter reorientation time with ketamine is further notable because ketamine has a longer duration of action than methohexital (10–15 minutes vs. 5–8 minutes),
19,37,38 a difference that would be expected to minimize a beneficial ketamine effect.
Although the neurobiologic substrate of diminished ECT-associated cognitive side effects with ketamine is unknown, some have suggested that it might be due to
N-methyl-
d-aspartate (NMDA) receptor antagonism.
2,13,17 It is known that ketamine is an antagonist at the NMDA subtype of glutamate receptor and that these receptors play a role in mediating long-term changes in synaptic connection strength, termed long-term potentiation (LTP), that are crucial for learning and memory.
14,16,39Several animal studies indicate a link between generalized tonic-clonic seizures, memory impairment, and hippocampal NMDA receptors.
13,15,39 These studies suggest that generalized tonic-clonic seizures, such as are induced with ECT, may lead to nonspecific, widespread physiologic changes that both temporarily diminish hippocampal capacity to sustain LTP (causing a relative anterograde amnesia) and disrupt the effects of prior LTP (causing retrograde amnesia). Such studies further suggest that, through NMDA antagonism, ketamine might limit cognitive dysfunction with ECT by preventing the widespread nonspecific hippocampal LTP that is otherwise induced during seizures and that appears to be cumulative over repeated seizure induction.
13,15,18 Further, NMDA receptor antagonism might prevent NMDA receptor activation due to factors other than the seizure that this literature also suggests may diminish memory dysfunction. The temporary prevention of LTP by ketamine might also be responsible for the anterograde amnestic properties of this medication that have been observed outside the context of induced seizures.
40,41 In the setting of induced seizures, however, it is possible that ketamine's ability to attenuate the seizure-induced LTP changes (which are more dysmnestic) might dominate, thereby resulting in a net protective effect on memory.
Side Effects of Ketamine Anesthesia With ECT
The finding that ketamine was associated with only easily tolerable and manageable postictal recovery phenomena and no cardiovascular side effects agrees with previous reports.
2,7,8,10,11 An exception is one subject who experienced persistent hallucinations for the day following ketamine anesthesia. This side effect has been seen with ketamine anesthesia in settings other than ECT and is consistent with its known hallucinogenic potential.
42 The individual who experienced this phenomenon was unique in receiving the highest dose of ketamine, 2.8 mg/kg (although prior studies have reported doses as high as 3.12 mg/kg without the occurrence of such symptoms
2) and because of both a history of recent alcohol abuse and the highest initial seizure threshold. It will require further studies to determine which patients are likely to experience such effects. For this reason, the possibility of hallucinatory side effects will have to be taken into account in the clinical decision to use ketamine anesthesia with ECT.
Rasmussen et al.
2 have hypothesized, on the basis of preclinical data,
43 that the relative absence of hallucinatory phenomena with the use of ketamine with ECT
2,7,8,10,11 may be due to depolarization and NMDA ion channel opening caused by the induced seizure, reversing the action of ketamine at the NMDA receptor. In the exceptional case noted above, an extremely high seizure threshold may have served to limit seizure expression, thereby diminishing the potency of the induced seizure to exert such an effect.
SUMMARY
We found that ketamine prolongs seizure duration and that this effect is particularly evident for those who have a less than 25-second seizure with methohexital anesthesia at the maximum available stimulus intensity. We also found evidence that ketamine enhances ictal EEG evidence of seizure intensity. These findings support the view that ketamine may have a less potent anticonvulsant effect than methohexital. Further, we found very preliminary evidence that ketamine may be associated with a lower level of ECT-related cognitive side effects than methohexital anesthesia. Lastly, our data indicate that ketamine is generally a safe anesthetic for use with ECT, although post-treatment hallucinations may occur. Based on these observations, it is reasonable to consider a switch to ketamine in the clinical setting when the capacity to elicit a seizure with methohexital anesthesia is limited even with the use of the maximum available stimulus intensity.
ACKNOWLEDGMENTS
Dr. Krystal received support from National Institute of Mental Health Grants K20 MH01151 and R01 MH57532. Dr. Krystal and Dr. Weiner are inventors listed on a U.S. patent that Duke University has licensed to MECTA Corporation. Neither receives royalties from this patent. The results of this study were presented in part at the 151st annual meeting of the American Psychiatric Association, Toronto, ON, Canada, May 30–June 4, 1998.