HIV-1 enters the central nervous system early in the course of infection and may produce a range of neuropsychological symptoms over time. These symptoms include impairments in psychomotor processing speed, executive function, and memory that may progress to clinical disorders, including minor cognitive motor disorder and dementia,
1 which independently predict shortened survival.
2 With the introduction of combination antiretroviral therapy (ART) that includes a protease inhibitor—or highly active antiretroviral therapy (HAART)—AIDS-related morbidity and mortality in populations with access to treatment have declined dramatically.
3 However, the impact of these therapies on CNS disease is in the early stages of investigation.
Recent evidence suggests that an elevation in CNS (cerebrospinal fluid and brain) viral load, but not in plasma viral load, is associated with HIV-related neurocognitive disorders.
4–6 HIV found in the CNS also exhibits genotypic and phenotypic variation compared with that found in the systemic circulation.
4,7 This evidence suggests that the CNS is an independent reservoir for HIV-1 replication, particularly in the later stages of disease.
8 This has important implications for ART, because sufficient blood-brain barrier penetration to achieve adequate HIV-1 inhibitory concentrations in the CNS may be necessary for durable antiretroviral efficacy against neurocognitive disorders.
9The nucleoside analog reverse transcriptase inhibitors (NRTIs) zidovudine, stavudine, lamivudine, and abacavir and the non-nucleoside reverse transcriptase inhibitors (NNRTIs) nevirapine and efavirenz have been found to penetrate the blood-brain barrier and achieve sufficient concentration to inhibit HIV replication.
9–13 Zidovudine (AZT) monotherapy at current standard and higher doses has been shown to improve neuropsychological performance
14,15 and severity of dementia
16 and was associated with the decline in the incidence of dementia from the late 1980s to the early 1990s.
17 However, the benefits of AZT monotherapy may be temporary because of the emergence of viral resistance.
18The protease inhibitors, with the exception of indinavir,
19 have poor blood-brain barrier penetration, which has generated concern that HAART might not confer any benefit in CNS disease. However, HAART regimens containing various protease inhibitors have been associated with reductions of CSF viral load to undetectable levels,
7,20 with reversal of white matter lesions on magnetic resonance imaging,
21 and with reversal of brain metabolic abnormalities detected by proton MR spectroscopy.
22 In a cross-sectional assessment of men with symptomatic HIV and AIDS, HAART, compared with less potent ART, was associated with superior performance on tasks of verbal memory, psychomotor speed, and executive function.
23 An open-label trial of HAART with 26 patients who had advanced HIV illness reported improvements in psychomotor speed and memory in 75% of patients after a 15-month follow-up.
24 Analysis of data collected over 1 year from 411 homosexual men in the Multicenter AIDS Cohort Study revealed that combination ART with or without a protease inhibitor was associated with improved psychomotor speed.
25In this study we aimed to further assess the pattern and durability of the potential neurocognitive benefits of progressively more potent ART after accounting for other factors that might affect cognitive performance, including practice—that is, the effect of repeatedly undergoing the battery of neuropsychological tests. The primary questions for this analysis are: Is progressively more potent ART associated with neuropsychological improvement over the course of 1 year? If so, what is the pattern of this improvement?
METHOD
Sample
Data for this study were derived from a longitudinal study of homosexual and bisexual men initiated in July 1995 to examine factors associated with psychosocial adjustment to AIDS.
26 Initially, 173 subjects were recruited from advertisements in community-based organizations. Although individuals at all stages of HIV infection were included, recruitment of those with symptomatic illness was emphasized. Men reporting a past or current history of intravenous drug use were excluded, as were men with insufficient English fluency to complete the testing. Laboratory evaluations, HIV symptom measures and illness staging, depression rating, and medication treatment data were collected at six semiannual visits over 2.5 years. Neuropsychological testing was initiated at visit 4 (18 months) with 141 subjects, and repeated at visits 5 and 6. The 141 subjects who were assessed at visit 4, which constitutes the neuropsychological baseline, are included in this analysis.
Measures
Laboratory measures. Assays to determine CD4 lymphocyte count and peripheral HIV RNA viral load (Roche, Amplicor; lower limit of detection 400 copies/ml) were performed by Corning-Metpath (now Quest) Laboratory.
Medical symptoms. A medical symptom checklist adapted from earlier HIV-positive cohort studies was used.
26 This self-report scale consists of 14 signs and symptoms commonly associated with HIV infection, such as night sweats and oral candidiasis. The total score, which ranges from 0 to 14, indicates the number of symptoms currently present.
Physical limitations. The Rand Physical Limitations Scale was used to assess physical functional ability.
27 This self-report scale requires respondents to rate their ability to accomplish ten physical tasks listed in descending order of difficulty. Higher scores indicate more physical limitations.
Depressive symptoms. The Beck Depression Inventory (BDI) was used as a self-report measure of depressive symptoms, with scores ranging 0 to 63.
28 Scores greater than 10 indicate clinically significant depressive symptoms.
Neuropsychological assessment. The neuropsychological test battery assessed the domains of attention, concentration, verbal memory, psychomotor speed, and executive function. Tests administered at visits 4 through 6 were the Trail Making Test, Parts A and B,
29 the California Verbal Learning Test (CVLT),
30 the Digit Symbol Substitution Test,
31 and the Stroop Color Word Test.
32 The Grooved Pegboard Test was administered at visits 4 and 6.
33 For data analysis, neuropsychological test scores were used in two ways: raw scores were used as continuous measures, and subjects were also rated as neuropsychologically impaired or unimpaired on the basis of their performance relative to published normative data. Subjects were classified as neuropsychologically impaired according to American Academy of Neurology criteria for HIV-associated cognitive motor disorders—that is, if they scored one or more standard deviations in the impaired direction relative to age-matched population-based norms on two or more neuropsychological tests or two or more standard deviations in the impaired direction on one or more neuropsychological tests.
1 Antiretroviral treatment potency. Given the naturalistic design of this study, the heterogeneity of ART regimens and the high rate of switching precluded analysis of the impact of stable regimens on cognitive performance. Thus, in order to provide a measure of regimen potency at each visit, five antiretroviral potency groups were established on the basis of recommendations of the International AIDS Society–USA Panel at the time of data collection.
34 Subjects were grouped as follows: category 1, zero to one antiretroviral; category 2, two NRTIs; category 3, three NRTIs + the original formulation of saquinavir (which had low bioavailability); category 4, two NRTIs + nevirapine or delavirdine (NNRTIs); and category 5, two NRTIs with or without one NNRTI + ritonavir, indinavir, or nelfinavir (HAART).
Procedure
Between July 1995 and December 1997, subjects were seen for six semiannual visits, each lasting 3 to 4 hours, during which self-ratings, neuropsychological assessments, and blood tests were completed. Medical information was elicited by clinicians under the supervision of a physician who worked in the hospital HIV/AIDS clinic (S.J.F.), and neuropsychological assessments were administered under the supervision of a board-certified neuropsychologist (W.v.G.). Written informed consent was obtained under the institutional review board guidelines of Weill Medical College of Cornell University. Subjects were paid $30 or $40 for each study visit.
Statistical Analyses
Outcome (dependent) variable distributions were examined for normality, and five variables were found to be significantly skewed. Log10 transformations were used for HIV RNA, Trail Making Test A and B scores, and Grooved Pegboard Dominant and Nondominant hand scores. Cross-sectional and preliminary longitudinal comparisons were made using t tests, χ2 tests, and analysis of variance.
The longitudinal data were fitted to mixed-effect models
35 with the SAS Institute's mixed procedure composed of fixed effect and random effect.
36 The data were fitted to the model with random effects of intercept and time, and unstructured covariance of random coefficients and constant error variances. The maximum likelihood method was used for the estimation. The type 3 F test was used in significance testing. In these analyses, all available data were used, without estimations for missing data. Subjects for whom there were missing data were retained in the analyses.
As a preliminary analysis to determine whether missing values resulting from dropout were missing at random, the logistic regression approach outlined by Ridout
37 and illustrated by Vonesh and Chinchilli
38 was applied. The results revealed that dropout was dependent on the observed outcome measurement history of the CVLT sum and long-delay free recall tests. In addition, time (measured as the number of semiannual visits) was correlated with all neuropsychological outcome measures, indicating informative censoring. Hence, for analyzing these variables, the conditional mixed-effects model representing a class of pattern mixture model
39 was employed. To take into account missing data, the subject's time in the study was used as a covariate for all measures. For the CVLT sum and long-delay free recall subtests, indicators representing death or other dropout or study completion and the number of missing visits for adjusting intermittent missing values were included as additional covariates in the model. In the modeling, change in individual response was focused, and subject-specific random effects were included in the model. Consequently, the intercept and time factor were assumed as random effects. For analyzing the dichotomous neuropsychologically impaired versus unimpaired outcome, as a linear function of covariates, we employed the generalized estimating equation (GEE) approach.
40 The analyses were performed with SAS PROC MIXED.
36In the longitudinal mixed-effects models, scores for neuropsychological tests at visits 4, 5, and 6 were the dependent variables. We used the following covariates to assess associations with neuropsychological test scores over time: number of visits, age, race, level of education, CD4 count, log10 HIV RNA, Physical Limitation score, HIV symptom score, BDI score, antidepressant or anxiolytic use (yes or no), and ART potency. With the exception of race and years of education, all covariates were time variant.
RESULTS
Demographics and HIV Illness Measures
At study baseline, the 141 subjects had a mean age of 40 (SD = 8), 59 (42%) were nonwhite (mostly African American or Hispanic), and 88% had some post–high school education (mean, 14 ± 2 years). Sixty-five (46%) were working more than 20 hours per week. Average time since notification of HIV status was approximately 5 years. Mean CD4 count at baseline was 249 ± 228 cells/μl; mean log10 HIV RNA was 4.9 ± 0.9 copies/ml; 115 (82%) of the subjects had AIDS; and nearly all subjects had symptomatic HIV illness.
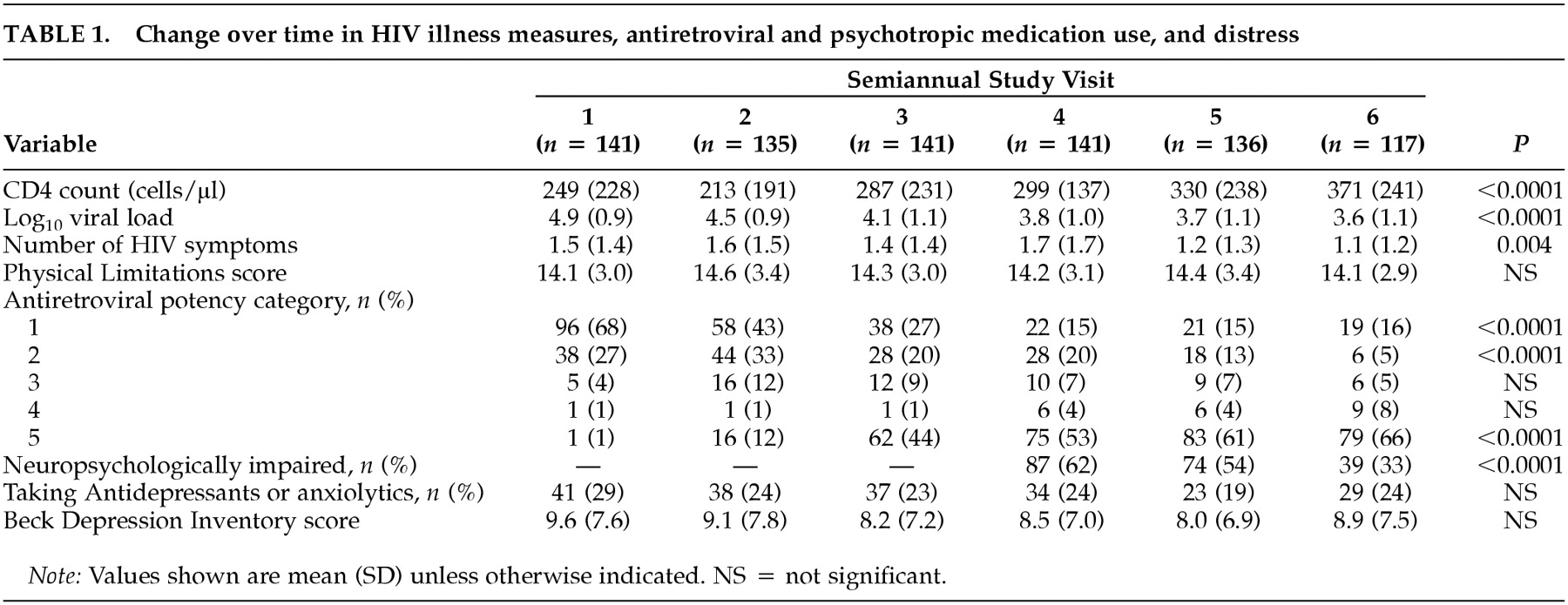
As
Table 1 shows, for the entire sample, mean CD4 count increased 122 cells/μl during the 2.5-year period, and mean log
10 HIV RNA dropped 1.3 log
10 copies. The mean number of HIV symptoms also dropped significantly. These favorable changes coincided with a dramatic rise in the number of subjects taking three or more antiretrovirals (potency categories 3, 4, 5), increasing from 6% at study baseline to 79% at time 6.
Between visits 4 and 6, 20 men dropped out of the study and four died. These subjects were more likely than those who remained to be neuropsychologically impaired at their last visit (71% vs. 36%, χ2 = 10, P<0.01), although they did not differ on other measures (data not shown).
Use of antidepressants and anxiolytics. Across the six time points, mean BDI scores were in the “not depressed” range and did not change significantly over time. Approximately 25 to 30% of subjects were taking antidepressants or anxiolytics, or both, at any given time point.
Neuropsychological function. As seen in
Table 1, nearly two-thirds of subjects had some neuropsychological impairment at baseline, and the impairment rate decreased significantly over the year of follow-up. At the final study visit, only 36% of subjects had impairment. At that time, subjects with neuropsychological impairment did not differ from those without on mean CD4 count (314 ± 255 vs. 381 ± 210,
P = 0.16), mean log
10 HIV RNA (3.7 ± 1.2 vs. 3.6 ± 1.0,
P = 0.53), or proportion with undetectable virus (33% vs. 49%,
P = 0.13). Sixty-seven percent of both the neuropsychologically impaired group and the unimpaired group were on HAART (potency category 5), and 81% and 77%, respectively, were taking any triple combination ART regimen (categories 3–5). Five (9%) of 54 subjects without neuropsychological impairment at baseline (three on HAART, one on saquinavir + two NRTIs and one on dual NRTIs) became neuropsychologically impaired by their last study visit. Under the multivariate GEE model, antiretroviral potency was not significantly associated with the dichotomous outcome of neuropsychologically impaired versus unimpaired (data not shown).
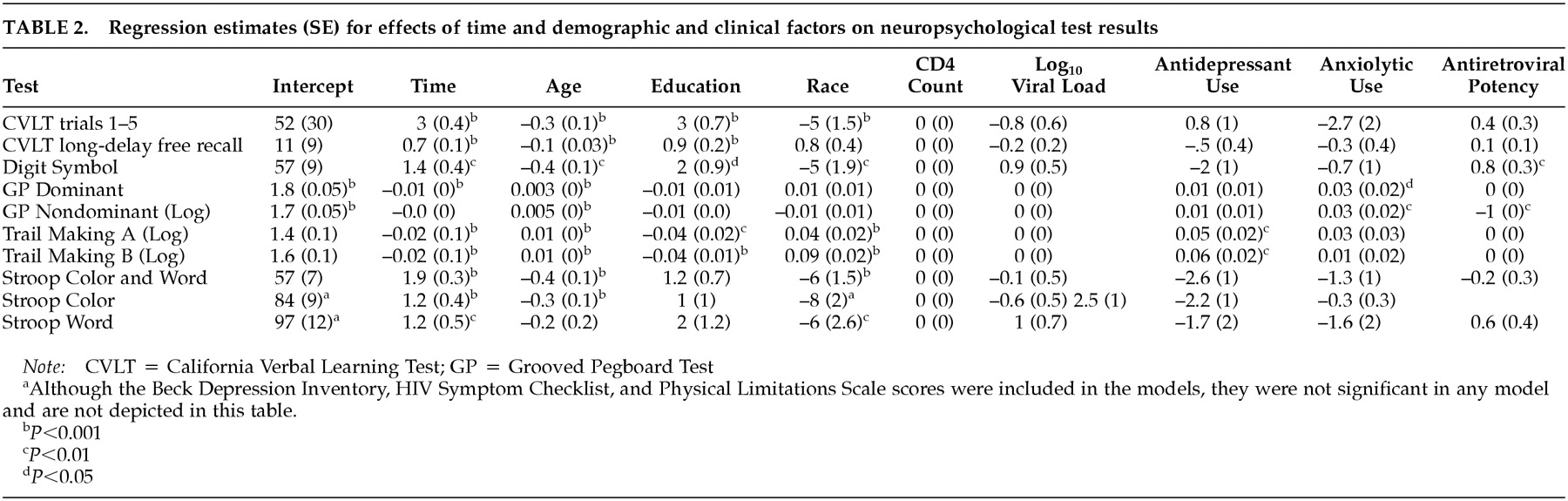
In contrast to the dichotomous outcome, in the mixed-effects models (
Table 2), after controlling for factors that might affect neuropsychological test performance over time, greater antiretroviral potency was associated with significant improvements in tests of psychomotor speed (digit symbol and grooved pegboard nondominant hand tests). Time (that is, over the course of repeated test administrations) was associated with improvement in neuropsychological test scores, and older age, lower education, and nonwhite race were consistently associated with poorer performance. Antidepressant use was associated with poorer performance on both parts of the Trail Making Test (psychomotor speed, cognitive flexibility), and anxiolytic use was associated with poorer performance on the grooved pegboard test (psychomotor speed). Depression, HIV symptoms, physical limitations, CD4 count, and viral load did not significantly affect neuropsychological performance.
DISCUSSION
In this longitudinal natural history study of HIV-positive men with symptomatic HIV and AIDS, the rate of neuropsychological impairment dropped by nearly 50% over the course of 1 year, but overall impairment was not related to ART regimen potency. However, longitudinal improvement in psychomotor speed was associated with progressively more potent ART, after controlling for multiple factors that might affect neuropsychological test performance, including practice from taking the tests repeatedly over time. Impairment in psychomotor speed is a cognitive domain consistently reported to be associated with HIV,
1 and improvement in this domain has been most consistently associated with ART.
15,23,25 Thus, subcortical brain structures mediating psychomotor speed may not only be sensitive to HIV-associated neuropathological changes but also to the benefits of progressively more potent ART.
Although the cohort as a whole experienced significant increases in CD4 count and reductions in plasma viral load with increasing use of combination ART, changes in neuropsychological performance were not associated with these HIV illness markers. This finding is consistent with the notion that CNS immunological and viral dynamics differ from those in plasma and that these markers are insufficient to monitor the impact of ART in the CNS. Thus, alternative markers, such as serial neuropsychological testing, CSF viral load measurement,
8 MR spectroscopy
22 and MR diffusion tensor imaging,
41 are more likely to be useful in monitoring the impact of ART in the CNS.
Although neuropsychological impairment was reduced by about 50% overall, more than one-third of this sample continued to be neuropsychologically impaired at the end of the study, and 9% of the patients who were unimpaired at baseline became neuropsychologically impaired after 6–12 months of follow-up. Moreover, about two-thirds of them had neuropsychological impairment despite being on HAART and one-third despite having plasma viral loads below detectable limits. The rate of neuropsychological test impairment seen in symptomatic HIV and AIDS before the advent of combination ART was found to exceed 50%.
42 In this cohort with symptomatic HIV and AIDS, after 1 year of follow-up, the prevalence of neuropsychological impairment approximated the median prevalence of impairment seen in asymptomatic HIV-positive samples before combination ART was used.
43 Residual neuropsychological impairment or the development of new neuropsychological impairment may reflect a number of factors that were not assessed in this study, including the irreversibility of HIV-associated neuropathological changes, variable CNS ARV penetration, variable adherence to the medication regimen, and the development of ARV-resistant HIV in the CNS. Such persistent neuropsychological impairment despite potent ART may impede functional ability, including capacity to work,
44 and may still constitute a risk factor for shortened survival.
2A number of limitations in this study might affect the generalizability of these findings. The sample did not include women and intravenous drug users. Given rapid changes in the availability and prescribing patterns of ART, it may be difficult to determine whether findings from the period of this study will be generalizable to the medications used currently or in the future. Finally, given the heterogeneity of ART and the high rate of ARV switching in this cohort, we were unable to compare the effects of different stable ARV regimens on HIV CNS disease. However, this naturalistic study very likely reflects the trajectory of CNS disease in the real-world context of variable ART.
This study contributes to the growing literature documenting the longitudinal benefit provided by potent ART for neuropsychological function, particularly psychomotor processing speed, in patients with HIV illness. In future research, studies that extend beyond 1 year of follow-up will be crucial in determining whether extended systemic improvement is accompanied by similar improvement in neurocognitive function.
ACKNOWLEDGMENTS
This research was presented in part at the NIMH conference on “HIV and the Nervous System: Emerging Issues” held in Washington, DC, in April 1999.