Hysteria, or conversion disorder, has been described since antiquity. The term
hysteria, based on the Egyptian theory of the wandering uterus, is credited to Hippocrates.
4 In 1859, Briquet described the
personnalité hystérique.5 The great French neurologist, Charcot, used hypnosis to elucidate and manipulate the clinical signs of the disorder. In the 20th century, Freud
6 proposed that an unconscious conflict is symbolically converted into a somatic symptom. To acknowledge this historical legacy, the terms hysteria and conversion will be used interchangeably in this article.
Formerly considered a dissociative disorder, conversion disorder is classified in DSM-IV-TR as a somatoform disorder along with somatization (Briquet's syndrome), pain disorder, hypochondriasis, and body dysmorphic disorder.
7 Psychogenic disorders of memory and personal identity are classified as dissociative disorders.
7 Both somatoform disorders and dissociative disorders are considered disorders of unconsciousness (i.e., not under voluntary control, unlike factitious disorder and malingering). Conversion disorder shares high comorbidity with anxiety, depression, and personality disorders.
The symptoms of hysteria can affect any aspect of elementary neurological function. including involuntary movements or paralysis, non-epileptic seizures, mutism, urinary retention, hallucinations, pain, blindness, deafness, and analgesia. Inconsistencies on examination suggest this diagnosis. These include simultaneous contraction of muscular agonists and antagonists, fluctuating weakness, non anatomical sensory loss, tunnel vision, and astasia-abasia. Some patients show a curious
belle indifférence toward their neurological handicap.
7 Brain imaging, electroencephalography, and sensory evoked potentials are normal. Although Slater
8 found that more than one-half of patients with hysteria would develop clear signs of neurologic disorder within 10 years of diagnosis, his claim has been contested recently.
9 Nevertheless, a diagnosis of conversion disorder does not rule out neurologic disease, and a diagnosis of neurologic disease does not rule out conversion disorder.
10Neuropsychological Studies
Pierre Janet
11 proposed that conversion disorder is a deficit of selective attention toward the pathological symptom. Janet emphasized the parallel between dissociation after overwhelming psychological trauma and hypnotically-induced alteration in consciousness. In psychoanalytical terms, conversion is the symbolic transformation of a dangerous emotion (aggression, rage, sexual excitement) into a somatic symptom, representing a compromise between the undesirable affect and the defense against it. Roelofs et al
12 reported more physical and sexual abuse and maternal unavailability in patients with conversion disorder compared to 50 patients with affective disorder, lending support to the proposed role of early emotional trauma in conversion disorder.
Ludwig
13 proposed that “selective corticofugal inhibition of afferent stimulation” operates in hysteria to exclude a somatic function from consciousness. C. Miller Fisher considered hysteria a delusion of somatic disability with lack of insight.
14 Flor-Henry et al
15 reported bifrontal and right posterior hemispheric cognitive dysfunction in 10 patients with hysteria compared to normal subjects, patients with psychotic depression, and patients with schizophrenia. Impairment of the dominant (left) hemisphere was more severe, reflected in subtle verbal imprecision, affective incongruity, and defective processing of endogenous somatic percepts, analogous to schizophrenia. Sackeim et al
16 showed that hysterically blind subjects, as well as subjects rendered blind by hypnosis, performed either better or worse than chance on visual recognition tasks, indicating that their choices were visually guided even if they were not consciously aware of seeing. Implicit visual processing may thus be disconnected from conscious awareness of vision.
16Perception is powerfully influenced by emotion and motivational state, which serve to enhance attention to salient stimuli.
17 Much of the pre-conscious processing of emotional stimuli involves connections between the amygdala-hippocampus and insular, orbital, and cingulate cortices which link perception, emotional state and memory.
17 The right hemisphere is theorized to be the locus of neural networks subserving attention to intra- and extrapersonal space, body schema, and personal identity.
18 Injury to this right hemispheric network is consistently associated with defects in emotion and feeling, as well as with anosognosia and neglect.
19 La belle indifférence may be a subtle form of neglect related to right hemisphere dysfunction. The right hemisphere may impart a negative bias to emotional experience.
20 These observations support Damasio's
21 hypothesis that hysteria involves defective mapping of the body state.
Functional Neuroimaging
There are to date few neuroimaging studies of hysteria. All of the studies described below are limited by small population size (the largest study includes only 7 patients); heterogeneous patient populations (e.g., motor versus sensory symptoms, non epileptic seizures or combined epileptic and non epileptic seizures); duration of the deficit (acute versus chronic); and presence of comorbid disorders such as depression or chronic pain. Overall they suggest variable alterations in the activity of specific cortical and subcortical areas may underlie conversion disorder, particularly prefrontal and parietal cortices, thalamus, and basal ganglia. To facilitate comparison of results, the studies have been grouped by state (resting, activated) and duration of deficit (acute, chronic).
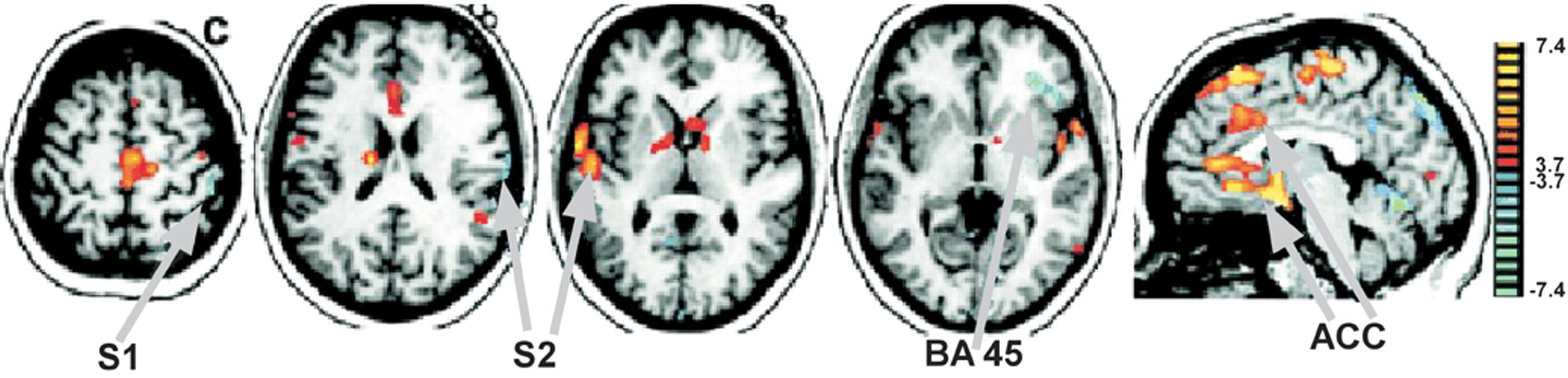
Initial sensory processing. An important finding in conversion disorder, as noted above, is that electrophysiological tests indicate that sensory and motor pathways are intact. Thus the question to be resolved is at what stage(s) does processing become abnormal. Magnetoencephalography (MEG) is a noninvasive electrophysiological technique that uses superconducting electrodes to measure the neuromagnetic fields generated by the brain's electrical activity. Hoechstetter et al
22 used MEG to record activity in primary (S1) and secondary (S2) somatosensory cortices in 3 patients with unilateral psychogenic motor and sensory loss (2 acute, 1 chronic) in response to tactile stimulation of the index finger of the affected and unaffected hands. Sixteen healthy subjects served as controls. S2 is activated about 30–50 msec after S1 and is involved in the early attentive processing of tactile stimuli. Both the patients and normal controls showed normal responses in both the contralateral S1 and bilateral S2 areas, regardless of the stimulated side. This is consistent with previous studies indicating that the early components of somatosensory evoked potentials are normal in conversion disorder and suggests that the altered processing that underlies the failure to perceive the stimulation occurs at a later stage of sensory integration than the initial activity in S2.
23,24 Resting (baseline) state of the brain. One study assessed the resting state of the brain in acute conversion disorder. Yazici and Kostakoglu
25 used
99mTc-HMPAO single photon emission computed tomography (SPECT) to measure regional cerebral blood flow (rCBF) in 5 patients with astasia-abasia (2 week – 1 month duration). The authors found decreased perfusion (0.72 – 0.88 of normal) involving only the left temporal lobe in 2 patients, left temporal and parietal lobes in 1, bilateral temporal lobes in 1 and only the left parietal lobe in 1. The authors note that the finding of left hemispheric involvement in all patients supports previous neuropsychological studies indicating more severe impairments of the dominant hemisphere in conversion disorder. They suggest that the bilateral perfusion deficit found in 1 patient may have reflected the persistence of conversion symptoms in the left leg during imaging. However, all patients had bilateral symptoms at the time of imaging. The role of left hemisphere impairment in conversion is controversial; other authors have found a higher incidence of left-sided motor and sensory deficits in patients with conversion disorder, hence implicating the right hemisphere.
26,27 A weakness of this study is that areas of abnormality were identified primarily by calculating the ratio between ipsilateral and contralateral regions of interest, with decreases of 10% or more considered significant. Thus more subtle or bilateral decreases may have been missed.
Response of the brain to sensory stimulation. Two SPECT studies found altered patterns of rCBF in response to sensory stimulation of the affected area during the acute stage of conversion disorder as compared to recovery. Tiihonen et al
28 used left median nerve stimulation in a single patient with left hemiparesis and paresthesia and comorbid major depressive disorder Median nerve stimulation is expected to increase contralateral parietal lobe rCBF. During the acute episode there was mild hyperperfusion (7.2%) in the frontal lobe and hypoperfusion (7.5%) in the parietal lobe contralaterally. After recovery (6 weeks later) rCBF in the contralateral parietal lobe was greater than ipsilateral. The results were interpreted as suggesting inhibition of somatosensory cortex by frontal cortex during the acute episode.
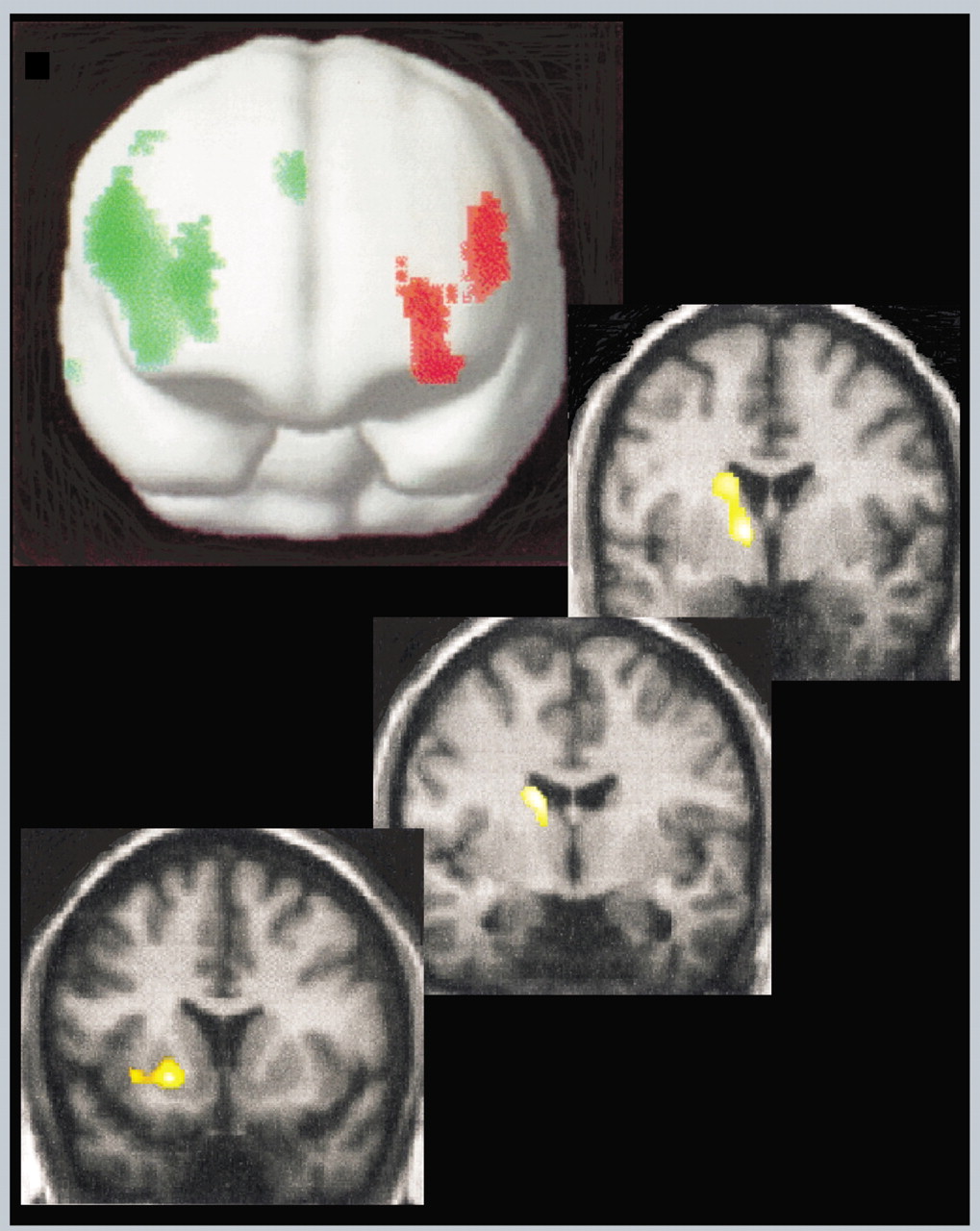
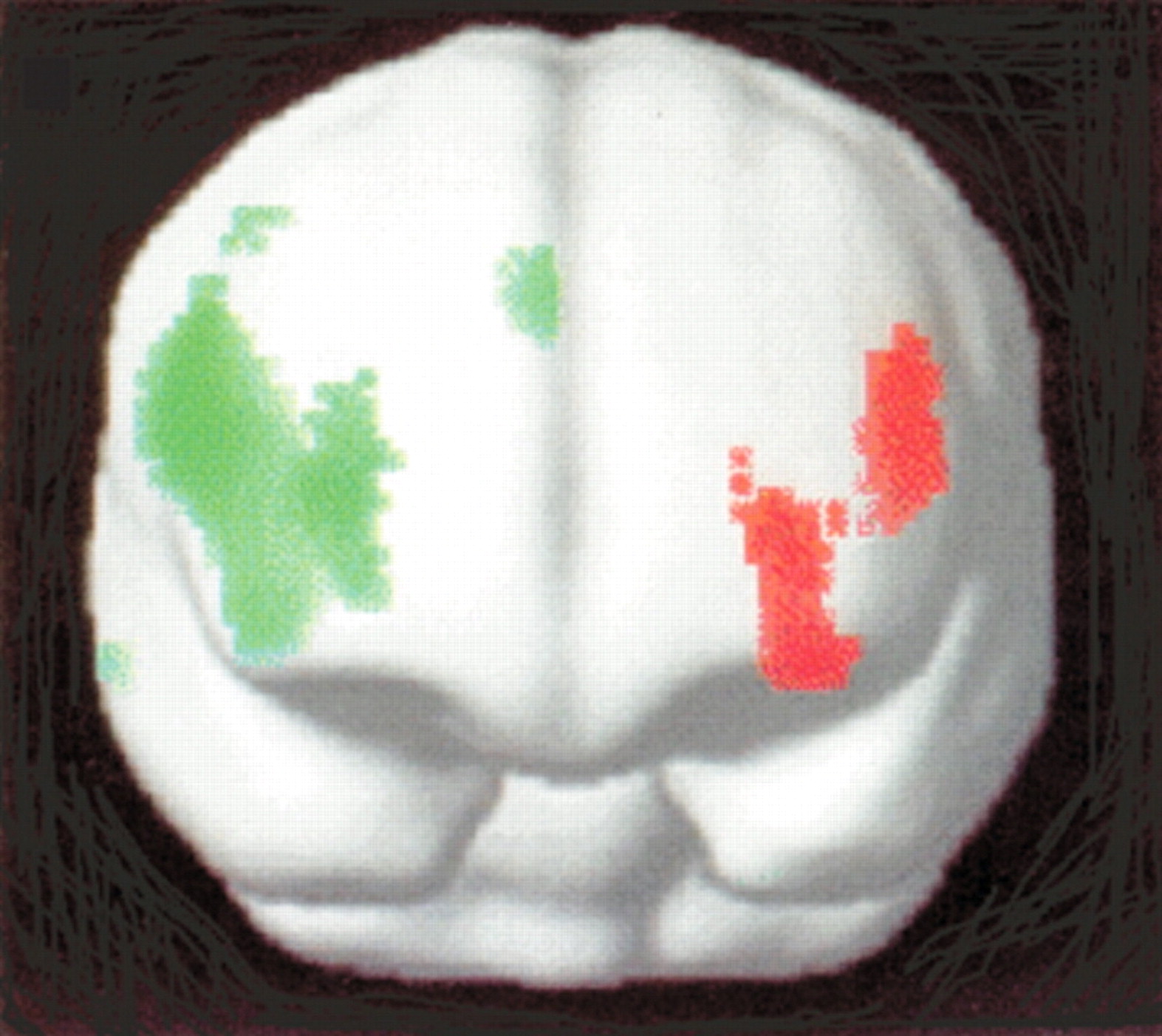
Vuilleumier et al
1 applied simultaneous vibratory stimulation to both affected and normal limbs of 7 patients during the acute stage of illness (less than 2 months; all patients displayed variable unilateral motor and sensory loss) and again 2–4 months later. By the second examination, 4 patients had completely recovered and 3 were still symptomatic. Two very different data analysis approaches were utilized, statistical parametric mapping (SPM) and scaled subprofile modeling (SSM). For SPM, analysis of covariance was applied on a voxel by voxel basis in order to identify areas that were significantly different in activation between conditions. In contrast to the previous study, no significant asymmetries in cortical rCBF were noted when rCBF was compared between the resting and stimulated state of acutely symptomatic patients. Comparison of rCBF in the stimulated state while patients were symptomatic and following recovery (4 patients) indicated decreased rCBF in the contralateral thalamus and basal ganglia that returned to normal. For SSM a type of principal component analysis was performed in order to identify networks of brain areas that changed together (covaried). Of particular interest was the grouping of contralateral thalamus, caudate and ventral lateral prefrontal cortex (BA 44/45, BA 11), supporting the results of the SPM analysis (
Figure 1). In addition, most (3/4) of the patients who recovered had a moderate increase in rCBF in dorsolateral prefrontal cortex on the right (contralateral for 2, ipsilateral for 1), resulting in the appearance of hypoactivation of the left dorsolateral prefrontal cortex similar to that reported by Spence in patients with chronic symptoms (to be discussed below).
3 An intriguing finding in the 3 patients with persisting deficits was that they had less activity in contralateral subcortical areas, particularly the caudate, at the first examination. Thus a finding of lower activation in these areas may predict poor recovery. The authors note that overall their findings support previous theories that attentional or motivational influences can modulate thalamus or basal ganglia to alter sensorimotor processes in conversion disorder. This is a salient study as it points to the involvement of subcortical structures in conversion disorder pathogenesis. The basal ganglia and thalamus participate in fronto-subcortical loops that modulate motor intention and sensory awareness under the influence of motivational state conveyed by reciprocal connections with the amygdala and orbitofrontal cortex.
29 These findings suggest disruption of sensory processing, movement, motivation, and attention at multiple nodes of a widely distributed network.
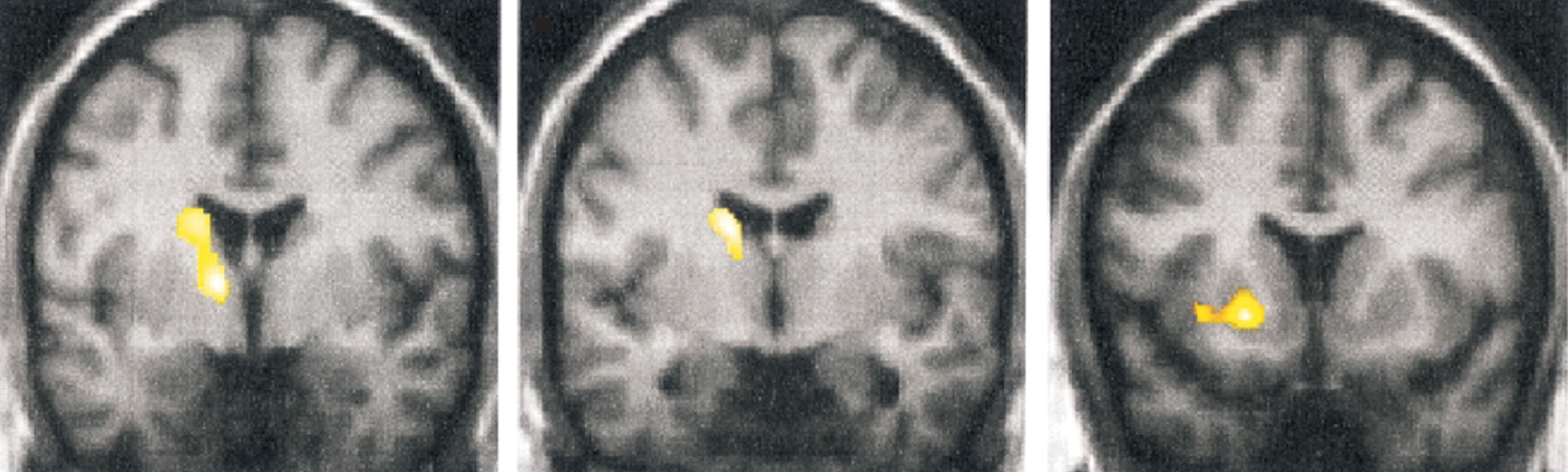
A third study used functional magnetic resonance imaging (fMRI) to look at patterns of activation in response to sensory stimulation in chronic conversion disorder.
2 Both innocuous (brush) and noxious (pressure) stimulation of either the affected (unperceived stimuli) or normal (perceived stimuli) limb were used in 4 patients with chronic pain and non dermatomal somatosensory deficits (NDSD).
2 All had variable motor loss in the affected limb, as well. Unperceived stimuli failed to activate areas that were activated by perceived stimulation, notably: anterior insula, thalamus, caudal anterior cingulate cortex, and ventrolateral prefrontal cortex (BA 44/45). This is similar to the network identified in the previous study. Unperceived stimuli were also associated with deactivation in primary and secondary somatosensory cortex (S
1, S
2), posterior parietal cortex and prefrontal cortex. Interpretation of deactivations in fMRI studies is presently controversial. Finally, unperceived (but not perceived) stimuli activated the rostral and perigenual anterior cingulate cortex (
Figure 2), an area not yet implicated in studies of patients with acute symptoms. The authors suggest conversion disorder may be a functional deafferentation due to active inhibition of somatosensory processing by limbic areas concerned with emotion and attention. This interpretation is compatible with that suggested by Tiihonen et al
28 and with Ludwig's
13 hypothesis that “selective corticofugal inhibition of afferent stimulation” operates in hysteria to exclude a somatic function from consciousness.
Response of the brain to attempted movement. Two studies using positron emission tomography (PET) found alterations in rCBF during attempted movement of the affected limb as compared to during movement of the normal limb in patients with chronic hysterical paralysis. Marshall et al
30 studied a single patient with hysterical left hemiparesis of 2.5 years duration. Voluntary movement of the unaffected right leg was associated with the expected increased rCBF bilaterally in dorsolateral prefrontal cortex, inferior parietal cortex, and cerebellum as well as contralateral primary sensorimotor and premotor cortex. Also as expected, preparation to move the normal limb increased rCBF in the same network with the exception of primary sensorimotor cortex. In contrast, both preparation to move and attempted movement of the affected limb failed to increase rCBF in contralateral primary sensorimotor cortex, although cerebellum was activated. In addition, rCBF increased in contralateral anterior cingulate and orbitofrontal cortices. The authors note that both these areas have been found to be important for suppression of inappropriate motor responses. These results are similar to the previous study, and consistent with the functional disconnection hypothesis postulated above, with limbic affiliated regions inhibiting or disconnecting cortical areas subserving motor planning and execution.
Spence et al.
3 used PET to elucidate the distinction between hysterical and feigned paralysis. They studied 2 patients with hysterical left arm monoparesis of 10 and 12 months duration and 1 with hysterical right arm monoparesis of 6 months duration. All 3 exhibited relatively decreased rCBF in the left dorsolateral prefrontal cortex during attempted movement of the limb in comparison to both controls and healthy individuals simulating paralysis by pretending difficulty with moving a limb (feigners). In contrast, decreased rCBF was found in the right anterior prefrontal cortex in feigners compared to both controls and patients (
Figure 3). The comparison done in the previous study (activations associated with normal movement versus attempted movement within a subject) was not reported. Since the left dorsolateral prefrontal cortex is involved in the programming and selection of motor patterns, dysfunction in the patients was interpreted as an abnormality of the higher components of volition.
Response of the brain during hypnotically induced paralysis. Halligan et al.
31 used PET to study rCBF in a right-handed subject with hypnotically induced paralysis of the left leg. When asked to move the “paralysed” leg, rCBF increased in the contralateral orbitofrontal and anterior cingulate cortices but not in the motor cortex. These results, virtually identical to Marshall et al.,
30 support the hypothesis of prefrontal inhibition of motor and sensory cortex and suggest that hypnotic paralysis may be a good model of hysterical paralysis, as proposed 100 years ago by Janet.
11 These two studies also lend themselves to an alternative interpretation. Instead of inhibition of motor and sensory cortex by limbic-affiliated regions, disconnection of intention from awareness may take place at the level of attention, producing a psychic blindness for sensation and movement analogous to anosognosia.
These studies, although they are preliminary in nature, involving small numbers of patients with different constellations and durations of symptoms, combine to support the hypothesis that conversion disorder is the result of dynamic reorganization of neural circuits which link volition, movement, and perception. Disruption of this network may occur at the stage of preconscious motor planning, modality-specific attention, or right fronto-parietal networks subserving self-recognition and the affective correlate of selfhood.
32 Activation of the anterior cingulate during conversion motor paralysis
30,31 and hysterical anesthesia
2 may reflect hyperattention to pain
2,33 or a site where inhibitory limbic input is conveyed to the motor cortex.
30,31 Alternatively, the anterior cingulate may be the “hidden observer”
34 whose activity reflects subliminal awareness of the conflict between intended action and outcome.
35–37Binding
The literature on binding may lend neurophysiological support to neuroimaging observations suggesting abnormal functional connectivity in conversion disorder. Binding refers to transient oscillatory hypersynchrony lasting hundreds of milliseconds among neurons in thalamus, posterior heteromodal association cortex and anterior brain areas involved in memory, motivation, and planning.
38 Rapid integration of activity among these distributed neural populations is achieved through iterative and highly parallel signaling at a frequency of 40 Hz. With respect to working memory, for example, reentrant interactions between frontal and parietal regions facilitate “the integration of the activity of spatially segregated brain regions into a coherent, multimodal neural process that is stable enough to permit decision-making and planning.”
38 Similar processes probably underlie conscious visual perception
39 and the conscious experience of free will.
40 In an auditory-visual associative learning paradigm, subjects who acquired awareness of the contingency schedule showed metabolic activation in a distributed network involving lateral prefrontal cortex, primary auditory and visual cortex, and medial cerebellum. As learning progressed, temporal coherence (binding) among these regions increased, suggesting that the involvement of prefrontal cortex, in interaction with other brain regions, is crucial for awareness.
41 Functional dysconnectivity, whether due to a structural brain lesion such as multiple sclerosis or head trauma, or to a sudden shift in neural state related to early adverse experience,
42 may disrupt conscious perception and voluntary control of movement.
38Brown and Marsden proposed that a primary role for the basal ganglia is to facilitate the binding of sensorimotor and dorsolateral prefrontal cortices and supplementary and cingulate motor areas in a coherent sequence of motor activity and thought. Slowness of voluntary movement, dystonia, and co-contraction of muscle agonists and antagonists are clinical hallmarks of basal ganglia dysfunction.
43 Bradykinesia, dystonia, and co-contraction of agonists and antagonists also occur in conversion disorder and may likewise reflect dysfunction of frontal-subcortical circuits.