Psychotic disorder following traumatic brain injury (PDFTBI) is reported to occur in 0.7%–8.9% of persons who sustain a head injury.
1 The latency between traumatic brain injury (TBI) and onset of psychotic symptoms is highly variable, with recent studies reporting a mean of 4–5 years.
2 PDFTBI is typically characterized by paranoid delusions and auditory hallucinations, with visual hallucinations and negative symptoms being less common.
3,4 An earlier TBI sustained in childhood or other congenital neurological disorders are reported to be risk factors for developing PDFTBI.
5Psychotic disorder following traumatic brain injury is of potential interest to clinicians and neuroscientists, as it may provide clues to understanding primary psychotic disorders such as schizophrenia.
6,7 For example, there is evidence indicating that the neuropathology of both PDFTBI and schizophrenia share similar locations.
8 Schizophrenia has been associated with abnormalities in temporal areas and frontal systems, the latter including the dorsal lateral prefrontal-thalamic-cerebellar loop.
9–11 Similarly, converging evidence from case studies reporting neuroimaging data,
3 state hospital inpatients,
8 psychiatric outpatients,
4 neurosurgery referrals,
12 a literature review of studies prior to 1969,
1 and brain-injured war veterans
13 report that the majority of PDFTBI cases demonstrate temporal lobe abnormalities followed in frequency by abnormalities of the frontal lobes.
2One implication of similarity in location of neuropathology in PDFTBI and schizophrenia is that psychosis due to frontal and temporal pathology may not be specific to schizophrenia, but it can result from certain changes in these brain structures caused by other neurological disorders.
2 Indeed, there is evidence suggesting that many secondary and nonschizophrenic psychotic disorders also affect frontal and temporal structures. Temporal and frontal abnormalities have been implicated in delusional disorders such as erotomania, the Capgras syndrome, and Cotard's syndrome
14–16 and have been found in the majority of cases of secondary psychosis including cerebral vascular accidents,
17 brain tumors,
18 and affective psychosis.
19 In addition, neurological disorders most commonly associated with secondary psychosis such as dementia of the Alzheimer's disease type and temporal lobe epilepsy are also associated with pathology to temporal areas.
4,20 In these disorders, there is some evidence suggesting that individuals who develop psychosis also demonstrate frontal pathology or a more generalized cerebral disturbance.
21–23In addition to sharing similar location of neuropsychopathology with schizophrenia, there is evidence suggesting that persons with PDFTBI demonstrate similarities in cognitive functioning. Neuropsychologically, schizophrenia has been associated with general cognitive deficits and lower intellectual abilities that are more pronounced in executive functioning, memory, and attention.
24,25 To date, there have been only two neuropsychological studies of PDFTBI. Both studies, however, describe a pattern of cognitive deficits that are very similar to those found in schizophrenia. In their review of case studies in the literature, Fujii and Ahmed
3 reported that 88% of cases with neuropsychological test data describe cognitive deficits, with 59% demonstrating memory deficits, while 41% demonstrate executive and spatial deficits. In a study comparing neuropsychological test data of subjects with PDFTBI and TBI without psychosis (TBIWP), significant differences were reported in executive functions, memory, parietal functioning, and language, with the PDFTBI group demonstrating poorer scores.
26 General intellectual functioning was not reported in these studies.
Although the aforementioned studies on PDFTBI with neuropsychological test data suggest that persons with PDFTBI share neuropsychological deficits similar to those with schizophrenia, these studies have methodological weaknesses that may make interpretation of the data questionable. First, the Fujii and Ahmed
3 findings were based on descriptions of case studies. There was no control group, and different tests were administered for each case. In the Sachdev et al.
26 study, the researchers used a comparison TBIWP group. However, the groups were not equivalent in verbal intelligence quotient (IQ). Thus test score differences may have been associated with premorbid cognitive deficits rather than the presence of psychotic condition. Additionally, the test data for this study were not uniform, as subjects were administered different batteries based upon the selection of the treating neuropsychologist. Instead, comparisons were made in cognitive areas that were judged to be normal or abnormal according to predetermined criteria.
Parallel to the implication of temporal and frontal pathology being common in psychotic disorders of different etiologies, similarities in neuropsychological functioning of different etiologies would provide support for a nonspecificity of presentation and symptoms in psychotic disorders. Evidence for this contention comes from studies examining neuropsychological test data of patients with different types of secondary and nonschizophrenic psychoses. For example, in a study comparing epileptic patients who had schizophrenia-like psychosis (SLPE) with schizophrenic patients, nonpsychotic epileptics and normal control subjects, Mellers et al.
23 found that the SLPE and schizophrenic groups had almost identical neuropsychological profiles with impairments in executive functioning, memory (verbal > visual), and attention. Two studies examined the neuropsychological profile of psychotic depression. Jeste et al.
27 compared neuropsychological test results in patients with psychotic depression, nonpsychotic depression, and schizophrenia. They found that the patients with psychotic depression were comparable to the schizophrenic group on neuropsychological measure but more impaired than the depressed group in learning, psychomotor speed, motor skills, and attention. Although traditional tests of executive functioning were not included, Trail Making Test, part B, which was described as a psychomotor speed task, can also be conceptualized as a test of executive functioning. In a study examining neuropsychological functioning in patients with psychotic depression, nonpsychotic depression, and normal control subjects, Schatzberg et al.
19 reported that the psychotic depressive patients demonstrated significantly greater impairment in verbal memory and the Stroop interference test than did the other groups, the latter being a measure of executive functioning. Finally, in a study examining neuropsychological profiles in erotomania, Fujii et al.
24 reported a cognitive profile characterized by impairments in executive functions and verbal memory.
The current study attempts to address weaknesses in previous studies examining the neuropsychology of PDFTBI by comparing uniform neuropsychological test data in the following groups: 1) PDFTBI; 2) TBIWP; and 3) persons with schizophrenia matched for gender, intellectual functioning, education, and age. It is argued that these findings would have implications for the nonspecificity of neuropathological location and neurocognitive presentation of psychotic disorders. Comparisons will be made between each group and norms from a normal sample. The following are our hypotheses: 1) all groups will demonstrate significantly lower scores when compared with the mean of the normative group, with the schizophrenic group demonstrating the most deficits and the TBIWP the least, while the PDFTBI group will fall in between. 2) Scores for the TBIWP group will differ from the PDFTBI and schizophrenia group, with the PDFTBI group demonstrating a neuropsychological profile that is similar to the schizophrenic group. Specifically, the PDFTBI group will demonstrate deficits in general IQ, attention, verbal learning, and executive functioning.
METHOD
Sample
Potential subjects for the PDFTBI group were state hospital inpatients referred for a neuropsychological evaluation within a 5-year period. Referrals for a neuropsychological evaluation were generally based on performance on an intake neurocognitive screening battery consisting of Trailmaking Test, parts A and B, and the Bender Gestalt. Referrals were also made if a known neurological condition such as HIV-positive status existed.
Criteria for patients with PDFTBI were based on the DSM-IV
28 criteria for psychotic disorder due to a general medical condition: 1) existence of hallucinations and delusions, 2) historical or laboratory evidence that the psychosis is the direct physiological consequence of the medical condition, 3) psychotic symptoms do not occur exclusively within the course of delirium, and 4) psychotic symptoms are not better accounted for by another mental disorder.
Due to cited difficulties in determining whether a psychotic condition is a direct consequence of the TBI,
7 additional criteria described by Cummings was adopted to further utilize DSM-IV criteria two for the disorder.
29 Thus, to insure the association between psychosis and TBI, PDFTBI subjects had to also meet the following criteria: 1) no reported family history of psychotic illness, 2) no prior history of psychotic illness, 3) no prior history of TBI, and 4) onset of psychotic symptoms after TBI.
A total of 261 patients were referred for neuropsychological testing during this period. Of the total, 24 patients met inclusion criteria for PDFTBI. All subjects were men. Eighteen were right-hand dominant, while six reported left-hand dominance. The sample was comprised of 11 Caucasians, 10 Pacific Islanders, and three Asians. Prior history of alcohol and methamphetamine abuse was reported in 13 and nine subjects, respectively.
The etiology of TBI was as follows: eight were sustained in motor vehicle accidents; six were from assaults; four were sustained in falls; three were from bicycle accidents; two resulted from boxing; and one sustained injury when a rock was thrown at his head. Twenty of the patients reported losing consciousness during the incident. Two denied losing consciousness, and two were not sure. Severity of TBI was based on criteria developed by the Mild Traumatic Brain Injury Committee of the Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine.
30 Criteria are based on duration of loss of consciousness (LOC). A head injury is classified as mild if the LOC is 30 minutes or less and moderate to severe if the LOC is longer than 30 minutes. In our sample, 16 patients sustained a mild head injury. Six were rated as moderate to severe, while the severity of the remaining two subjects was not known.
The TBIWP group consisted of outpatient referrals made to the same clinic during the same time period. Eleven women and 10 men were in the group. Among this group of men and women, 16 of them were right-hand dominant, and five had mixed-hand dominance. Five had a history of alcohol abuse, while five reported histories of methamphetamine abuse. There were nine Caucasians, four Asians, four Pacific Islanders, two African Americans, and two Hispanics. The etiology of head injuries included 14 motor vehicle accidents, three falls, three gunshot wounds, and one assault. According to the aforementioned criteria, seven sustained mild head injuries, and nine sustained injuries that were moderate to severe. Data for five cases were not available. None of the subjects in the PDFTBI or TBIWP groups were currently experiencing seizures.
The schizophrenia group consisted of inpatients diagnosed with schizophrenic spectrum disorder referred to the same department during the same time period. Schizophrenic patients who had a history of TBI were eliminated from the study. Of the 24 subjects, 21 were men and three were women. Twenty-two reported right-hand dominance, while two were left-hand dominant. This sample was comprised of nine Caucasians, seven Pacific Islanders, four Asians, and four African Americans. Nine subjects reported past alcohol abuse, while six reported methamphetamine abuse. Of the 24 patients, 16 were diagnosed with paranoid schizophrenia, and seven received diagnoses of chronic undifferentiated schizophrenia, while one was diagnosed with schizoaffective disorder.
Measures
The design of the study was a retrospective chart review. Results from the following tests were extracted from the files of the neuropsychology department: Wechsler Adult Intelligence Scale (WAIS),
31,32 the immediate recall of the Anna Thompson story from the Logical Memory subtest,
33 Trail Making Test parts A and B,
34 verbal fluency,
35 and Wisconsin Card Sorting Test (WCST).
36 These tests were selected for their significance to functional outcome according to a meta-analysis of the literature by Green et al.
37 In addition, the WAIS Block Design and Similarities subtests were selected, as previous studies reported spatial and language deficits in PDFTBI subjects.
31,32Means for normal samples in age and education were taken from standardization samples
33,36 or samples similar to the ones we used.
38–40Statistical Analysis
Analyses included chi-squares, one-sample t tests, and analyses of covariance (ANCOVAs). Significance level was set at 0.05. Independent t tests were conducted to examine significant results of the ANCOVA.
RESULTS
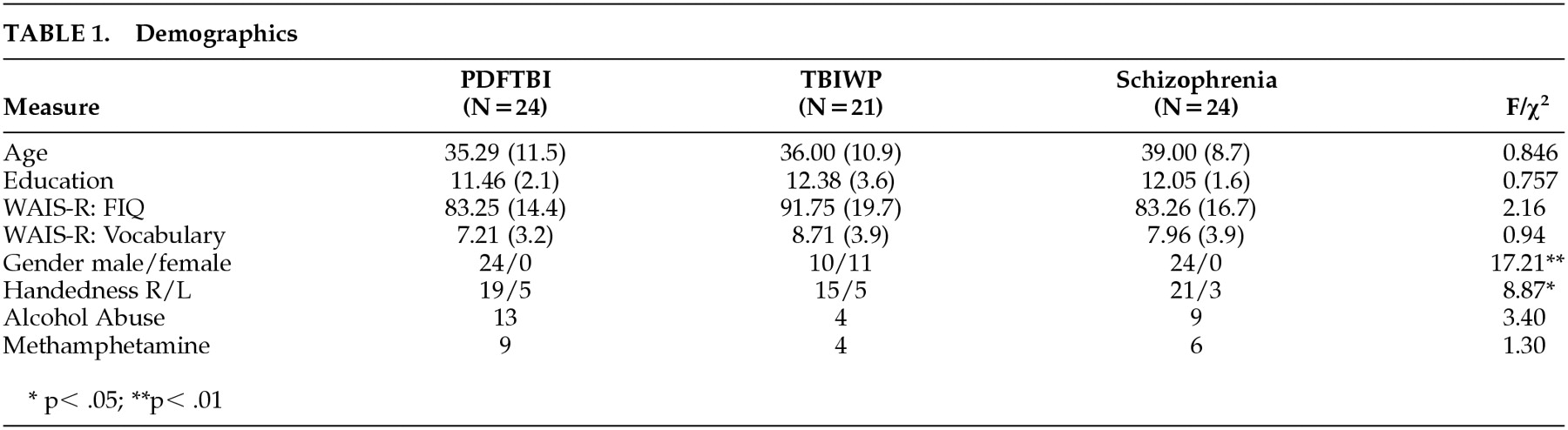
Data for comparisons of demographics are presented in
Table 1. The groups did not differ with regard to age (
F=0.846, df=2, 66,
P>0.05), education (
F=0.757, df=2, 66,
P>0.05), WAIS full-scale IQ (
F=2.16, df=2, 66,
P>0.05), WAIS vocabulary score (
F=0.94, df=2, 66,
P>0.05), abuse of alcohol (χ
2=3.4, df=2,
P>0.05), or abuse of methamphetamine (χ
2=1.30, df=2,
P>0.05). Although not significant, a weak trend was demonstrated with regard to WAIS full-scale IQ (
F=2.16, df=2, 66,
P=0.12). Significant differences were found for gender ratio (χ
2=17.21, df=2,
P<0.01) and handedness (χ
2=8.87, df=2,
P<0.05). The TBIWP group comprised the most women, while the schizophrenic group had the lowest percentage of left- or mixed-hand-dominant subjects. To test whether our significant group comparison was affected by between-group differences in gender or hand dominance, t tests were conducted on the entire sample, with gender and hand dominance as independent variables. No significant differences were found for any dependent variable.
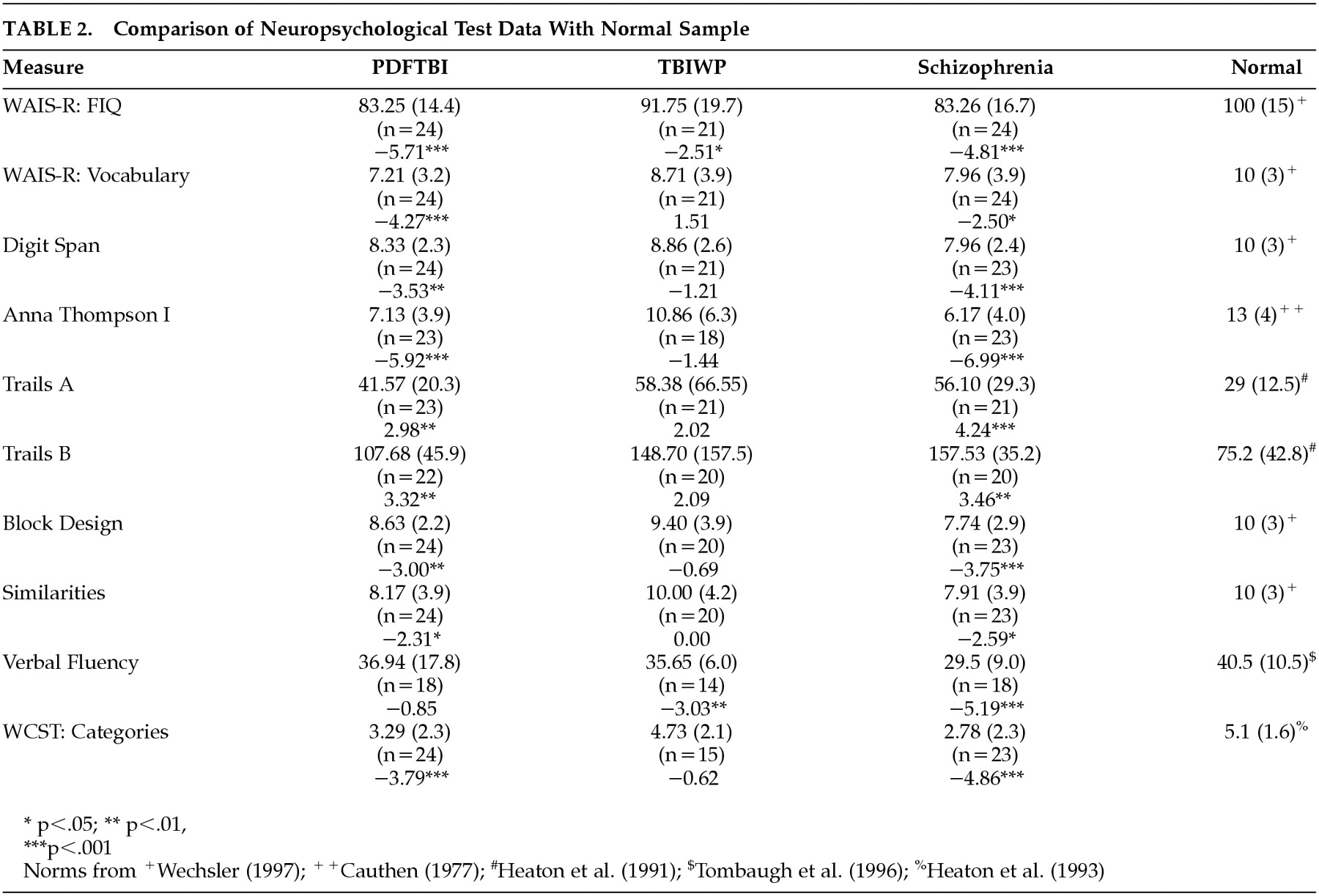
To determine whether each group differs from normal subjects, one-sample t tests were conducted between each group and the mean for normal samples. These data are presented in
Table 2. As predicted, the schizophrenic group demonstrated the most significantly lower neuropsychological scores when compared to means of normal samples. Significant differences were found for all neuropsychological measures. After the Bonferroni correction (corrected p=0.0017), however, only the following measures remained significant: WAIS full-scale IQ (
t=−4.81, df=22,
P<0.001), Digit Span (
t=−4.11, df=22,
P<0.001), Anna Thompson story (
t= −6.99, df=22,
P<0.001), Trail Making Test, part A (
t=4.24, df=20,
P<0.001), Block Design (
t=−3.75, df=22,
P<0.001), verbal fluency (
t=−5.19, df=17,
P<0.001), and the WCST (
t=−4.86, df=22,
P<0.001).
For the PDFTBI group, all measures were significantly lower than the mean for normal subjects, with the exception of verbal fluency (t=−>0.85, df=17, P>0.05). However, after applying the Bonferroni correction (corrected P=0.0017), the only significant measures included WAIS full-scale IQ (t=−5.71, df=23, P<0.001), WAIS vocabulary (t=−4.27, df=23, P<0.001), Anna Thompson story (t=−5.92, df=22, P<0.001), and the WCST (t=−3.79, df=23, P<0.001). For the TBIWP group, scores on the WAIS full-scale IQ (t=−2.51, df=20, P<0.05) and verbal fluency (t=−3.03, df=13, P<0.01) were significantly less than means for a normal sample. However, none of these measures remained significant following the Bonferroni correction.
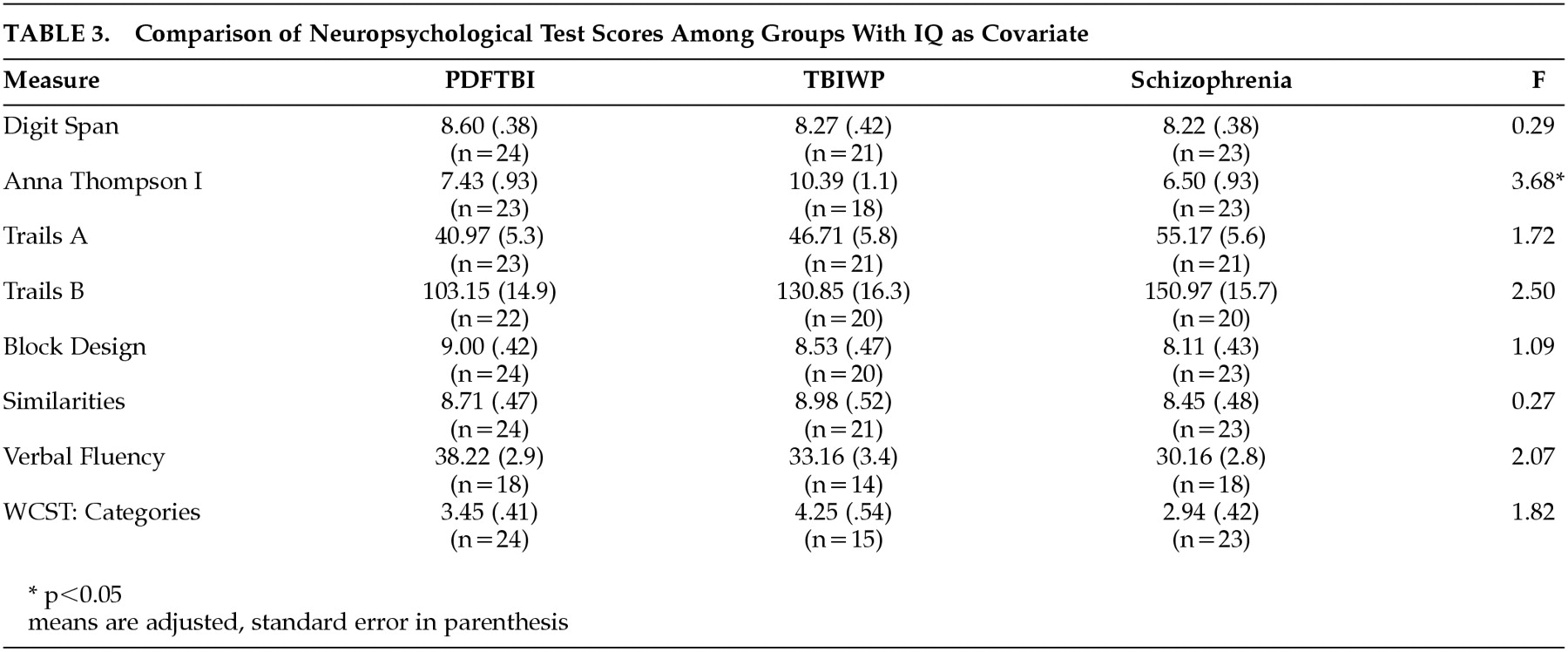
Due to the trend in WAIS IQ, ANCOVAs were conducted with IQ as a covariate to parse the effect of IQ on statistical comparisons. The results of these analyses are presented in
Table 3. The only significant finding was a difference on the Anna Thompson story (
F=3.68, df=2, 61,
P<0.05). This finding, however, was not significant after the Bonferroni correction (corrected
P=0.006). The number of significant differences between the three groups (PDFTBI, TBIWP, schizophrenic) versus the normal sample was statistically significant (χ
2=6.54, df=1,
P<0.0).
DISCUSSION
Our findings provided mixed support for our hypotheses. All groups demonstrated significantly lower neuropsychological test scores when compared with the means of normal samples, with the schizophrenic group demonstrating the most deficits and the TBIWP group the least. Counter to our predictions, however, the TBIWP did not demonstrate any significant differences from the normal subjects after the conservative Bonferroni correction.
Our second hypothesis that the neuropsychological profile of the TBIWP would differ from PDFTBI and schizophrenia groups was only partially supported as well. Support for this position was demonstrated by the lack of differences between the TBIWP group and the normal subjects combined with multiple significant findings between the normal sample and the PDFTBI and schizophrenic groups. Yet, when compared head to head, only the Anna Thompson story was significant. This difference, however, was no longer statistically significant after the Bonferroni correction.
Our prediction that the PDFTBI group would demonstrate a pattern of deficits similar to that of the schizophrenic group, including significantly lower intelligence, attention, verbal learning, and executive functioning, also received partial support. In comparison with normal means, the PDFTBI group demonstrated significant lower scores in intelligence (WAIS IQ), memory (Anna Thompson), vocabulary, and executive functioning (WCST). The PDFTBI was not as globally impaired as the schizophrenic group, although the aforementioned pattern of deficits is consistent with the literature on PDFTBI, which indicates that the most common deficits include memory and executive functioning, and the pattern is also consistent with reported spatial and language deficits
3,26 and the literature on schizophrenia, which indicates a general decline in overall abilities with the most severe deficits in executive functioning, memory and attention,
24,25 as compared with the normal group. In addition to impairments in the aforementioned areas that have been replicated in the literature,
24,25 the schizophrenic group was impaired in visual spatial functioning (Block Design), working memory (Digit Span), and other tests associated with executive functioning (verbal fluency and Trail Making Test, part A).
The more global deficits of the schizophrenic group can likely be attributed to differences in neuropathology. The neuropathology of TBI varies with type and severity of injury. The most common type of head injury for the general age range of our sample was closed head injury (CHI), secondary to motor vehicle accidents, and then assaults. In CHI, the primary injuries are contusions at the site of impact and contra coup lesions on the opposite side of the brain. For acceleration-deceleration injuries from motor vehicle accidents, lesions to frontal and temporal areas from the surrounding bony structures and shearing and tearing of white matter are common. In most cases, these lesions are mild and superimposed upon a brain that has developed normally, prior to the TBI.
41In contrast, converging evidence suggests that schizophrenia is a neurodevelopmental disorder, with lesions that may originate in utero.
42 One of the most common findings on computerized tomography/magnetic resonance imaging (CT/MRI) are enlarged ventricles that are believed to be associated with atrophy in the mesial temporal structures.
43 Imaging studies also demonstrate atrophy in the frontal lobes, thalamus, basal ganglia, and cerebellum.
10,11 Atrophy in frontal areas have been associated with reduced neuropil, most commonly found in the dorsal lateral areas.
9 Temporal areas are associated with a disarray of neurons.
44 Thus, not only is the neuropathology likely to be more consistently extensive in schizophrenia, but the presence of early abnormalities can affect the normal development of cognitive abilities.
45 Given these differences, it is not surprising that the schizophrenic group demonstrated a more global pattern of deficits.
For the PDFTBI group, deficits on specific tests may provide additional clues to the neuropathology. Vocabulary, which is associated with left hemispheric functioning, would suggest abnormalities in the dominant hemisphere. This pattern would be consistent with psychosis as a thought disorder.
46 Moreover, one of the most common findings in schizophrenia is hypofunctioning of the left dorsal lateral area while performing the WCST.
47,48Similarly, normal performances of the PDFTBI group are consistent with differences in associated symptoms between PDFTBI and schizophrenia. In schizophrenics, performance on verbal fluency has been associated with the severity of negative symptoms.
37 In contrast, negative symptoms have been found to be relatively rare in PDFTBI when compared to schizophrenia.
3,26 Thus, it is not astonishing that verbal fluency in the PDFTBI group would not be significantly different from that of normal subjects.
Although neuropsychological test data generally have poor localization value, specific deficits can provide clues to abnormal cerebral functioning. The pattern of neuropsychological deficits are consistent with the location of neuropathology for both disorders, as memory disorders are most commonly associated with temporal areas, while executive functioning and attention are most commonly associated with frontal areas.
41In summary, although our specific hypotheses received only partial support, our findings suggest that the neuropsychological profile of patients with PDFTBI differ from that of TBIWP patients in that the PDFTBI group demonstrated more impairment in select areas. Specific cognitive areas include deficits in general intelligence, verbal memory, executive functioning, and vocabulary. The first three cognitive areas are similar to those found in schizophrenic patients, while the latter may suggest left hemispheric dysfunction, which is also common in schizophrenia.
47,48Findings from our study provide further evidence of similarities in the neuropsychological profiles of psychotic disorders with different etiologies. Deficits in executive functioning and verbal memory have been observed in studies examining neuropsychological performances in psychotic depression,
19,27 schizophrenia-like psychosis of epilepsy,
23 and erotomania.
14These deficits parallel findings that temporal and frontal abnormalities are common in psychotic conditions that are secondary to neurological disorders such as dementia of the Alzheimer's disease type,
28 temporal lobe epilepsy,
4 cerebral vascular accidents,
17 brain tumors,
18 affective psychosis,
19 and delusional disorders.
14–16Similarities in the emerging literature on the neuropsychology and localization of neuropathology in psychosis that is secondary to neurological disorders and schizophrenia argue for the nonspecificity of both cognitive deficits and localization of cerebral abnormalities in psychotic disorders of different etiologies. Additional evidence, including replication studies with larger samples sizes and neuropsychological studies with other secondary and nonschizophrenic samples, is needed to cogently argue for the robustness of this association. If additional support is found, one implication is that it may be useful to alter the general manner of conceptualizing psychosis as being particularly germane to schizophrenic spectrum disorders. Instead, psychosis may be conceptualized as a neurocognitive-behavioral syndrome that is similar to aphasia or amnesia that results from specific brain pathology or abnormal circuits or interactions between cerebral structures. This manner of thinking may facilitate the conceptualization of heterogeneous disorders such as schizophrenia, as different symptoms can be parsed by associating them with abnormalities to different structural or neurochemical components that contribute to their manifestation. This manner of analysis would be similar to classifying the different types of aphasias according to their corresponding structural abnormalities.
There are several limitations to our study that may have impacted the results or affect the generalization of our findings. Foremost are sampling differences for each group. Both the PDFTBI group and the schizophrenic subjects were taken from neuropsychological referrals at a state hospital inpatient setting based on poor Trail Making Test and Bender Gestalt scores. In contrast, the TBIWP group involved outpatient referrals. Thus it is possible that differences between the TBIWP group and the others were due to sampling versus actual differences in the general population. We attempted to control for sampling differences by matching groups on age, education, and IQ. However, our statistical matches may not have controlled for differences in functional impairment. Related sampling issues are: 1) the retrospective nature of the data collection may have introduced biases in the sample; 2) the small sample size may have resulted in findings unique to this sample; 3) although there were no significant differences in the number of substance abusers within each group, substance abuse, particularly methamphetamine, may have affected test results of specific individuals, and thereby impacted the group results; 4) number, amount, and type of medications were not controlled (psychotropic medications can either enhance or impair cognition); 5) although not likely to have affected our results, there were significant differences between groups in handedness and gender ratio; and 6) normative samples consisted primarily of Caucasians, whereas there were many subjects of Asian/Pacific Islander ancestry in our samples.
Despite these limitations, our study could be used as a guideline for further research exploring similarities and differences between psychotic disorders of different etiology. To improve upon our methodology, future studies should employ prospective designs with larger samples taken from more equivalent populations. The potential confounding effects of comorbid substance abuse should also be addressed. Additionally, more neuropsychologically pure tests such as eye-tracking and backwards masking could be used to control for the confounds of multiple functions associated with the more common neuropsychological tests used in this study.