Over the past decade, there have been numerous investigations dealing with the nature and prevalence of cognitive dysfunction in HIV-infected individuals. There is clear agreement that cognitive impairment is more common in patients with more advanced infection. Although there has been considerable controversy, there is an emerging consensus that a small proportion of patients with early-stage infection may also have subtle cognitive impairment that may be sufficient to interfere with normal daily functions.
1 Numerous additional investigations have begun to isolate the factors associated with the identification or prevalence of these impairments.
Previous investigations have demonstrated that age, education level, a history of AIDS defining opportunistic infections, and rate of immune decline are associated with a greater risk of impairment.
2–5 In contrast, depression does not appear to be consistently associated with greater impairment in HIV infection. Preliminary data from our laboratory have indicated that higher anxiety levels are associated with cognitive impairment in HIV-infected patients. Recent data have demonstrated that alcohol use has an additive effect on cognitive impairment in HIV infection.
7Most studies of the impact of drug use in HIV-infected individuals have been conducted in subjects with intravenous drug abuse.
8 To our knowledge, there have been no studies examining the impact of other types of drug abuse on cognitive function. Marijuana use is relatively common among populations at risk for HIV infection.
9 Aside from recreational use, marijuana has medicinal benefits such as analgesia, increasing appetite, and decreasing nausea. Nevertheless, previous studies have demonstrated that the active component of marijuana suppresses immune function
10 and may have an additive effect with already immunocompromised systems,
11as with HIV infection.
Studies of acute marijuana use have demonstrated effects on cognitive function, but the impact of chronic marijuana use on cognitive function is controversial. Some studies have reported evidence of adverse effects,
12–14 while others have not.
13,15–18 Pope et al.
13 reported an effect of current heavy use on the recall of a word list until day 7, but no reported effect 28 days following the last use. Significant effects have also been reported for various aspects of both learning and memory. However, the residual cognitive effects of chronic marijuana use remain poorly understood.
19Cannabinoid receptors have been found in the hippocampus, which are activated by marijuana consumption.
20 This activation reduces presynaptic neurotransmitter release below what is needed for long-term potentiation, which reduces long-term changes in synaptic strength. This is presumed to be related to glutamate-mediated inhibition of calcium channels.
21–23 Marijuana use has also been demonstrated to influence brain morphology and cerebral blood flow (CBF). Intravenous infusion of delta-9-tetrahydrocannabinol (THC) produced increased CBF in the frontal regions bilaterally as well as the insula, cingulate gyrus, and subcortical regions.
24 Smoking marijuana resulted in increased CBF in orbital and mesial frontal lobes, insula, temporal poles, anterior cingulated and cerebellum. Reduced CBF was noted in temporal auditory regions, visual cortex, and regions related to attention. No significant alterations were noted in the hippocampus, basal ganglia, or nucleus accumbens.
25 Subjects who began using marijuana prior to age 17 have been shown, with PET scan, to have smaller whole brain and percent central gray matter compared to individuals who began use after that age.
26 In contrast, there is no evidence of atrophy or global change in tissue volume using MRI in young adults with current frequent marijuana use.
27Therefore, it was of interest to examine the potential effect and interaction of marijuana use on cognitive function in HIV-infected people. Subjects were stratified by disease stage and extent of marijuana use. In view of the potential confounding effects of age, depression, anxiety, and comorbid alcohol use, these variables were included as covariates.
METHODS
Subjects
The sample consisted of three groups: 74 seronegative healthy subjects, 127 HIV-positive subjects who were asymptomatic, and 87 HIV-positive symptomatic subjects [56 Centers for Disease Control (CDC) stage B and 31 CDC stage C]. There was no attempt to select subjects with subjective complaints of cognitive impairment. Individuals with a history of intravenous drug use, head injury resulting in unconsciousness for more than 60 minutes, neurological disorder, or learning disability were excluded. Both control and HIV-positive subjects were informed of the study through registration with an AIDS Clinical Trials Unit (ACTU), through local HIV-related community support groups, by newspaper advertisements, or by word of mouth. Subjects were provided with an informational brochure describing the study. All subjects received detailed descriptions of the nature and purposes of the study, and all gave written informed consent. Blood samples were drawn during the subjects' regularly scheduled ACTU visit, which corresponded to the time of enrollment in the current study. Prior to their enrollment in this study, subjects were followed as part of a cohort in the ACTU. Serostatus was determined by enzyme-linked immunosorbent assay, and positive assays were confirmed by Western blot.
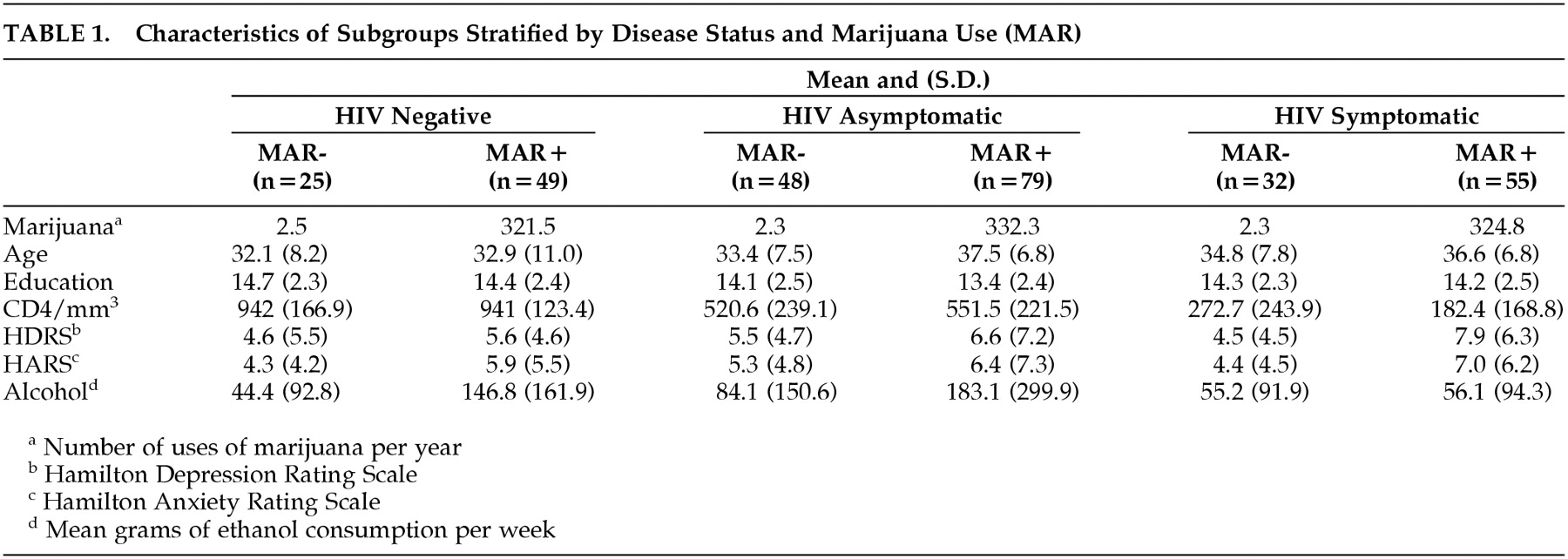
Subjects were also stratified on marijuana use based on the distribution of the entire sample. Marijuana use was based on self-report over the past 12 months in a semiquantitative questionnaire. Subjects who used marijuana less than once per month composed the no/ minimal use group, and subjects who reported marijuana use at least once per week composed the frequent use group. Within the no/minimal use subgroups, 84 (45.6%) denied any use of marijuana within the past year. As seen in
Table 1, the mean annual use (uses per year) of marijuana in the no/minimal use group was approximately 2.5, whereas in the frequent use group, subjects used marijuana almost daily (approximately 330). Subjects whose marijuana use fell between the selection criteria were excluded to ensure separation between the marijuana use subgroups. The demographic characteristics were analyzed using a 3 × 2 analysis of variance (disease stage by marijuana use). As seen in
Table 1, the groups were similar on age and education, but there were significant differences among the groups on depression, anxiety, and alcohol use. Therefore, these variables were included as covariates in the analysis of neuropsychological performance.
Procedures
Neuropsychological Measures: Subjects completed an extensive neuropsychological battery that included standardized neuropsychological measures and a series of simple and choice reaction-time measures. Because the nature of cognitive changes in HIV-infected people has been characterized as a subcortical dementia, tests were selected on the basis of demonstrated sensitivity to the effects of subcortical dementia in general and HIV infection in particular. The following standardized neuropsychological measures were used: Wechsler Adult Intelligence Scale-Revised, Selective Reminding Test, Visual Memory Span Forward and Backward from the Wechsler Memory Scale-Revised, Verbal Concept Attainment Test, Wisconsin Card Sorting Test, Verbal Fluency, Figural Fluency, Trail Making A and B, Grooved Pegboard, and the Paced Auditory Serial Addition Test.
28 Simple and choice reaction-time measures were also included because these tasks have consistently been found to be sensitive to the effects of HIV infection. A summary performance score was determined for each subject, consisting of the number of measures on which the subject performed 1 standard deviation (SD) or more below the mean of controls. In addition to the neuropsychological measures, subjects completed the Hamilton Depression Rating Scale and the Hamilton Anxiety Rating Scale. Subjects also completed a semi-quantitative self-report that yielded data on the frequency and quantity of marijuana and alcohol use. The data used for these analyses were based on mean self-report of use over the year prior to participation.
RESULTS
The data were analyzed using a 3 × 2 analysis of variance (disease status × marijuana use). As noted above, for age and education there were no significant main or interaction effects. However, on the Hamilton Depression Rating Scale there was a significant effect of marijuana use (
F = 8.98, df = 1,276,
p < 0.003). Subjects with frequent marijuana use reported more symptoms of depression. On the Hamilton Anxiety Rating Scale, there also was a significant effect of marijuana use (
F= 8.58, df = 1,276,
p < 0.004). Once again, subjects with frequent marijuana use reported more symptoms of anxiety. It was also considered that subjects with frequent marijuana use might also be prone to frequent use of other drugs such as alcohol. As shown in
Table 1, for weekly consumption of alcohol, there was a significant effect of disease stage (
F= 4.14, df=2, 276,
p < 0.013), for marijuana use (
F=12.41, df=1,276,
p < 0.001), and a trend toward a significant interaction (
F=2.67, df=2,276,
p <0.071). Subjects with symptomatic HIV infection and subjects with infrequent marijuana use used significantly less alcohol. There was no difference in current or past use, abuse, or dependence of other drugs. Therefore, depression, anxiety, and alcohol use were included in the analyses as covariates. Comparison of CD4 levels revealed the expected group differences. Among the symptomatic subjects, there were no significant effects of marijuana use on CD4 levels and no interaction. Thus, any interactions of disease stage and marijuana use cannot be attributed to differences in level of immunosuppression.
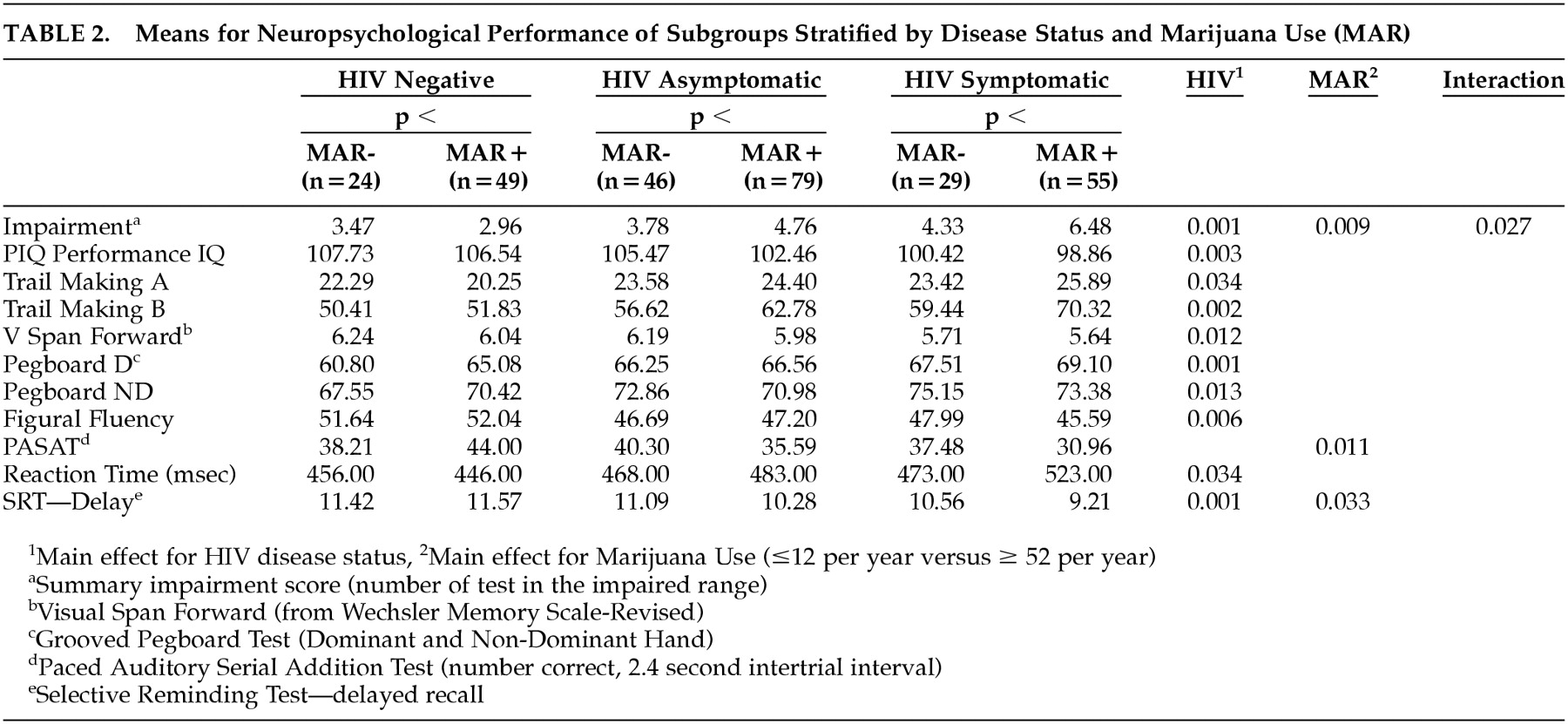
The initial analysis was based on the overall impairment score, which reflected the total number of measures on which an individual achieved a score that was one standard deviation below the mean of the control group. As shown in
Table 2, after controlling for the effects of depression, anxiety, and alcohol consumption, there was a significant effect for disease stage (
F=7.18, df=2,276,
p <0.001), a trend toward a marijuana effect (
F=3.49, df=1,276,
p < 0.063), and a significant interaction effect (
F=3.53, df=2,276,
p < 0.031). Inspection of the data indicates that the difference between the no/minimal and frequent marijuana use groups was greatest among the subjects with symptomatic HIV infection.
The data were further analyzed to determine which areas of neuropsychological performance were affected by marijuana use. This was accomplished by a series of analysis of covariance (ANCOVAs) controlling for the effects of depression, anxiety, and alcohol consumption. The results of these analyses are also presented in
Table 2, which demonstrates that the effect of marijuana use was reflected only on a measure of delayed memory, on which there was also a significant interaction effect. Analysis of simple main effects revealed that the differential impact of marijuana was progressively greater in relation to increasingly severe HIV disease. There were no differences on other measures of attention, learning, or memory related to marijuana use.
DISCUSSION
These data provide support for the hypothesis that frequent marijuana use may be associated with cognitive dysfunction in the context of more advanced HIV infection. Although the main effect for marijuana use only approached significance, there was a significant interaction, suggesting that frequent marijuana use was associated with greater cognitive impairment among subjects with symptomatic HIV infection. This effect appeared to be primarily related to performance on memory tasks. These results cannot be attributed to the influence of confounding variables such as depression, anxiety, or alcohol use, all of which were included as covariates in the data analysis.
These results are partially consistent with previous studies that have reported poor memory function in frequent marijuana users.
12 The frequent users in this study used marijuana on almost a daily basis, which is comparable to the level of use in previous studies. However, the effect of marijuana was only found in those subjects with advanced HIV disease. There was no evidence of a significant marijuana effect in the HIV-negative control subjects or in subjects with asymptomatic HIV infection, although the difference between marijuana use groups increased across stages of HIV disease. The lack of difference in cognitive function in the healthy subjects, and to some extent the asymptomatic HIV-infected subjects, is consistent with previous studies that provide no clear evidence of residual cognitive deficit associated with chronic marijuana use when assessed in nonacute situations. It should be noted that the frequent use group used marijuana on nearly a daily basis, and some subjects reported multiple episodes of use per day. It is unclear if there is a threshold for marijuana use that is related to cognitive impairment, but the extent of marijuana consumption in these subjects suggests that high levels of marijuana consumption may not lead to residual cognitive dysfunction. It is similarly unclear if the interaction of marijuana use and HIV disease stage would be demonstrated in subjects with frequent but more modest use.
These data also support the hypothesis of an interactive effect of marijuana use and HIV disease on cognitive function. The data revealed numerous effects of disease stage, but the effects of marijuana use were most prominent in the subjects with symptomatic infection. This is consistent with previous studies
7 which have indicated that the subtle effects of other conditions may be more manifest in the setting of advanced immune suppression. Although the effects of marijuana use are somewhat controversial, there are some studies that suggest an impact of chronic abuse on cognitive function. The present study suggests that these subtle effects are more likely to be expressed in subjects with greater immune suppression. The mechanism for this interaction is unclear. Since both HIV infection and marijuana have known immunologic effects, it is possible that these combined effects may have a synergistic effect on immune decline. It is unclear whether there are specific immune markers or processes that are central to cognitive dysfunction.
These data also have implications for patient management. If chronic marijuana use does indeed exacerbate the cognitive dysfunction associated with HIV infection, it may be important to encourage subjects to reduce their level of consumption. However, this may be in conflict with the management of other symptoms associated with HIV infection. Since some HIV infected patients use marijuana for control of nausea and appetite, reduction in marijuana use may lead to increase in these symptoms. Individuals with continued chronic marijuana use should be alerted to the possibility of greater memory dysfunction, and could be encouraged to use memory books or other assistive devices to circumvent their memory problems. Further investigation is needed to determine if these apparent adverse effects of marijuana are associated with less frequent use and to determine the underlying mechanism.
ACKNOWLEDGMENTS
This study was supported by grants from the National Institute on Drug Abuse (DA10248), the National Institute of Mental Health (MH45649), and the National Institute On Alcohol Abuse and Alcoholism (AA11720).