Point and lifetime prevalence of major depression are about 15% and 50%, respectively, in patients affected by multiple sclerosis (MS).
1 Independent of the yet unresolved etiopathogenetic issues, antidepressant psychopharmacologic interventions are often needed in the clinical management of patients.
However, no clear-cut guidelines to choose drugs and dosages can be derived from literature. Tricyclic antidepressants were widely used, but a placebo-controlled trial of desipramine showed modest beneficial effects, with side effects limiting dosage in one-half of the patients.
2 Studies on selective serotonin reuptake inhibitors (SSRIs) are scarce, and some have questioned the efficacy of sertraline,
3while others have reported a risk of exacerbating MS symptoms with fluoxetine.
4Fluvoxamine is a SSRI of proven efficacy in major depressive disorder. In the present multicenter (n=8) open-label study, we evaluated the efficacy of fluvoxamine, administered at a fixed dose for 3 months, in a homogenous group of patients affected by major depression associated with MS and who received a stable fixed dose treatment with interferon β-1b.
METHODS
Key inclusion criteria were a confirmed diagnosis of MS with a relapsing-remitting disease course; a diagnosis of major depressive episode based on the Structured Clinical Interview for the DSM IV, in the absence of other psychiatric diagnoses; stable medical conditions; and the willingness to be administered fluvoxamine. A written informed consent was obtained by each patient after study procedures were fully explained.
Severity of depression and of MS-linked neurological impairment were rated at baseline and at weeks 2, 4, 8, and 12 by administering the Montgomery-Åsberg Depression Rating Scale
5 (MADRS) and the Expanded Disability Status Scale
6 (EDSS). Since the EDSS, which is based on a standard neurological examination, is insensitive to cognitive dysfunction in MS, we assessed neuropsychological status at baseline by means of Mini-Mental State Examination (MMSE), Milan Overall Dementia Assessment (MODA), and Wechsler Memory Scale (WMEM).
A validated Italian version was used for all measures.
7 Ratings were performed by trained raters. Whenever possible the same rater conducted admission and follow-up ratings for each patient.
Changes in outcome measures (MADRS and EDSS scores) over time and the possible effects of baseline neuropsychological measures on improvement were analyzed with random effect regression using all available panel data.
8 This statistical method includes all the data of all subjects on whom postbaseline information is available, whether or not they completed the trial or were judged adequately exposed to treatment, thus allowing a more realistic analysis of longitudinal data. Response to treatment was categorically defined as a 50% reduction in MADRS scores.
Forty-three participants (14 men and 29 women; mean age=38.4±8.6 years; interferon β-1b dosage=8 million IU every other day; mean baseline MMSE score= 28.9±1.4; mean baseline MODA score=92.6±7.7; mean baseline WMEM score=86.0±14.6) were included. None of the patients had a positive previous psychiatric history, nor had first-degree relatives affected by mood disorders.
RESULTS
Fluvoxamine was started at 50 mg/day and slowly up-titrated by 50 mg every 5 days until a dose of 200 mg/ day was reached. After 3 months 36 patients continued treatment and seven (16.3%) dropped out because of side effects (all because of gastrointestinal problems: nausea, vomiting, dyspepsia). Categorical response rate among completers was 19/36 (53%) after 1 month, 28/36 (78%) after 2 months, and 31/36 (86%) at endpoint. Among noncompleters 3/7 (43%) had responded to treatment before dropping out.
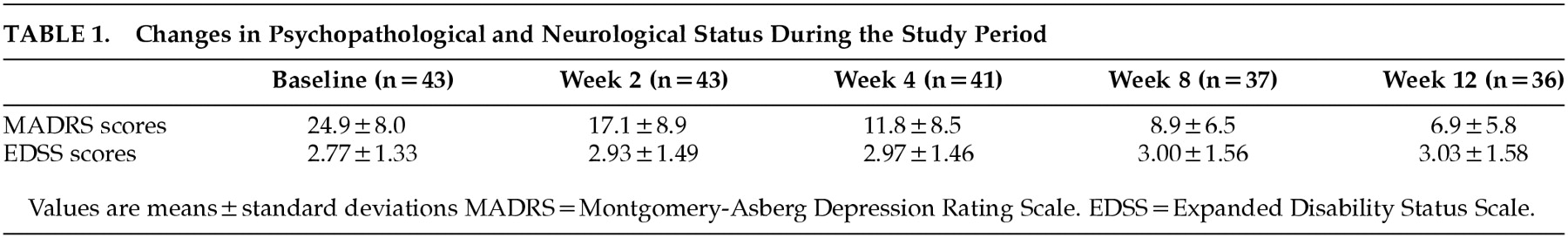
Changes in MADRS and EDSS scores during treatment are shown in
Table 1.
Severity of depression substantially improved during treatment. Random regression analysis detected a highly significant improvement in MADRS scores (estimate=−7.22; SE=0.36; Z=−20.1; P<0.00001), irrespective of treatment center (estimate=−0.59; SE=0.55; Z=−1.08; P=0.280). Improvement was unrelated to basal neuropsychological status (MMSE: estimate= −0.49; SE=0.37; Z=−1.32; P=0.186; MODA: estimate=−0.06; SE=0.13; Z=−0.49; P=0.628; WMEM: estimate=−0.05; SE=0.08; Z=−0.61; P=0.542). Basal MS-linked impairment, as rated on EDSS, influenced mood improvement, but the observed direction of effect was unexpected: higher EDSS scores, better response to treatment (estimate=−2.12; SE=0.95;Z=−2.23; P= 0.026). It should be noted that EDSS and MADRS scores were not correlated at baseline (Pearson's r=0.01, P= 0.945).
Age marginally influenced response (older age, lower improvement: estimate=0.25; SE=0.13; Z=1.92; P=0.055), while sex did not (estimate=−2.31; SE= 1.91; Z=–1.21; P=0.225).
No patient showed a critical exacerbation of MS symptoms, but EDSS scores marginally worsened during the study period (estimate=0.008; SE=0.004; Z=1.92; p=0.055). The lack of statistical significance makes uncertain the meaning of these changes, which could have become significant had treatment continued. However, the EDSS mean scores at baseline and at endpoint were both in the low range of the scale (from minimal to moderate disability, with full ambulatory function).
DISCUSSION
This is the first trial to examine the effects of fluvoxamine for the treatment of major depression associated with MS. Despite a 16.3% attrition rate, 34/43 (79%) patients achieved response. It is possible that slower titration and the use of flexible dose regimens may improve tolerance and decrease dropouts.
From a neurological point of view, the drug treatment appeared to be well tolerated during the period of time necessary to achieve psychopathological response. Patients in our sample had a current mild to moderate disability linked to MS, which was not significantly worsened by treatment. Safety of treatment in patients with severe neurological disability needs further research.
Estimates of drug efficacy based on open trials always need placebo-controlled confirmations. It should be noted, however, that studies available in natural history suggest that depression associated with MS is not self-limiting when untreated.
9 Thus, the present results warrant clinical interest for fluvoxamine treatment of MS-associated major depression. A placebo-controlled replication of the present findings is anticipated.
ACKNOWLEDGMENTS
Participating centers and investigators include Prof. P. Bramanti and Dr. A. Prudente from the Chair of Neurophysiopathology, Centro Neurolesi, University of Messina; Prof. A. Reggio and Dr. M.R. L'Episcopo from the Institute of Neurological Sciences, University of Catania; Prof. E. Montanari and Dr. L. Ludovico from the Division of Neurology, Ospedale Civile, Fidenza; Prof. G. Costantino and Dr. T. Carrella from the Division of Neurology, Ospedale Civile, Foggia; Prof. R. Cotrufo, Dr. A. Menditti, and Dr. G. Lus from the Chair and II Division of Neurology, Primo Policlinico, University of Napoli; Prof. C. Florio and Dr. O. Campese from the Division of Neurology, Ospedale Cardarelli, Napoli; Dr. P.B. Carrieri and Dr. L. Lavorgna from the Department of Neurological Sciences, University of Naples; Prof. D. Caputo and Dr. R. Cavarretta from the IRCCS S. Maria Nascente, Milan.