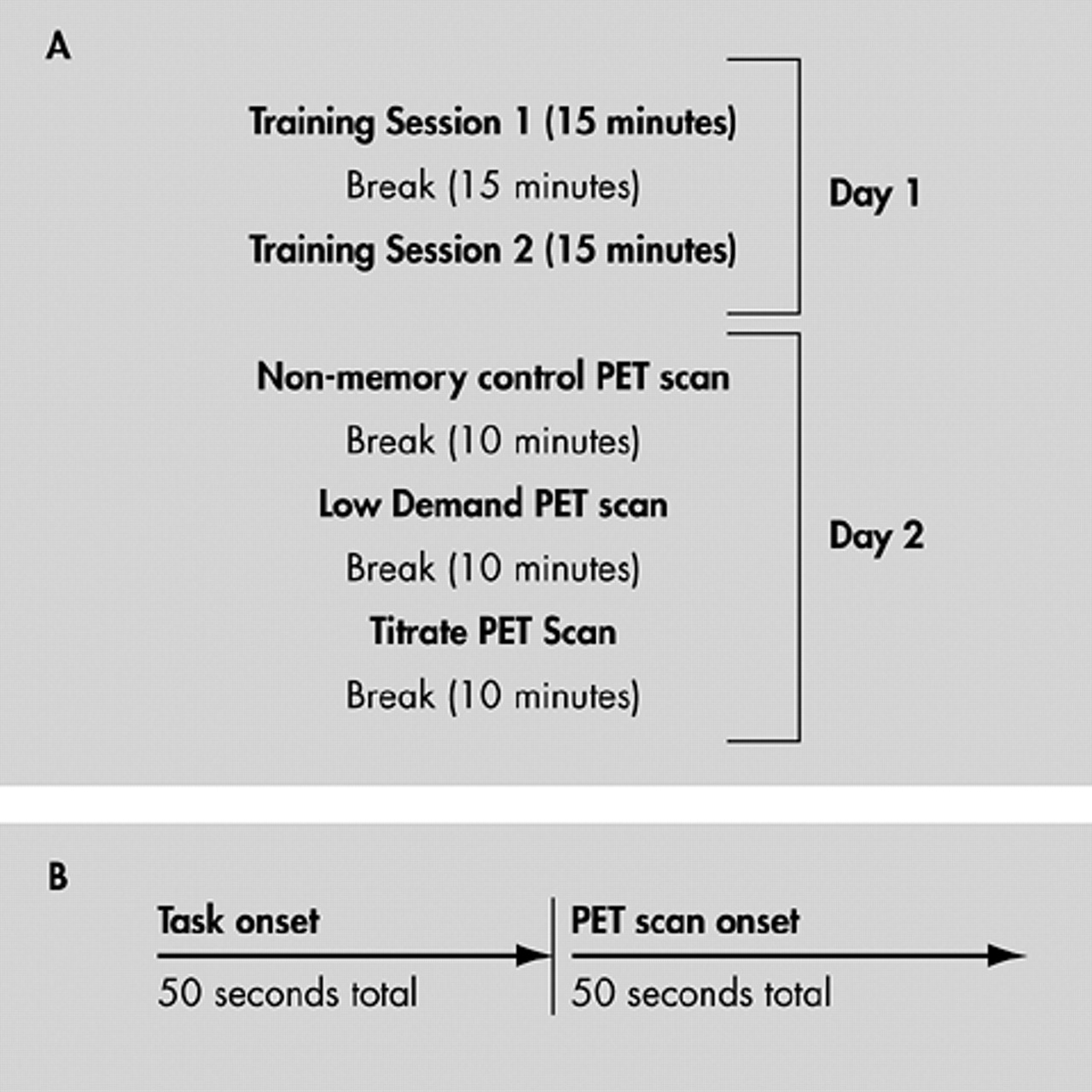
Nonverbal memory change with normal aging has not been as extensively studied as verbal memory, and most studies have not corrected for task difficulty differences across young and elderly subjects. The current study used a continuous, nonverbal, recognition task that attempted to match all subjects for level of difficulty. We hypothesized that, relative to younger subjects, elderly subjects would show more brain blood flow changes during the continuous recognition task performance, especially in frontal lobe regions. These changes would reflect attempts by the elderly subjects to maintain performance in the face of changes related to normal aging.
DISCUSSION
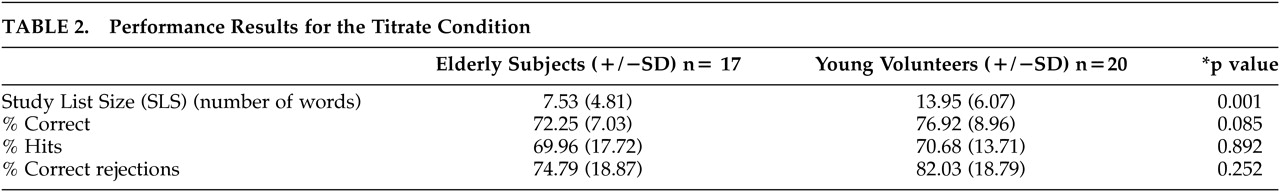
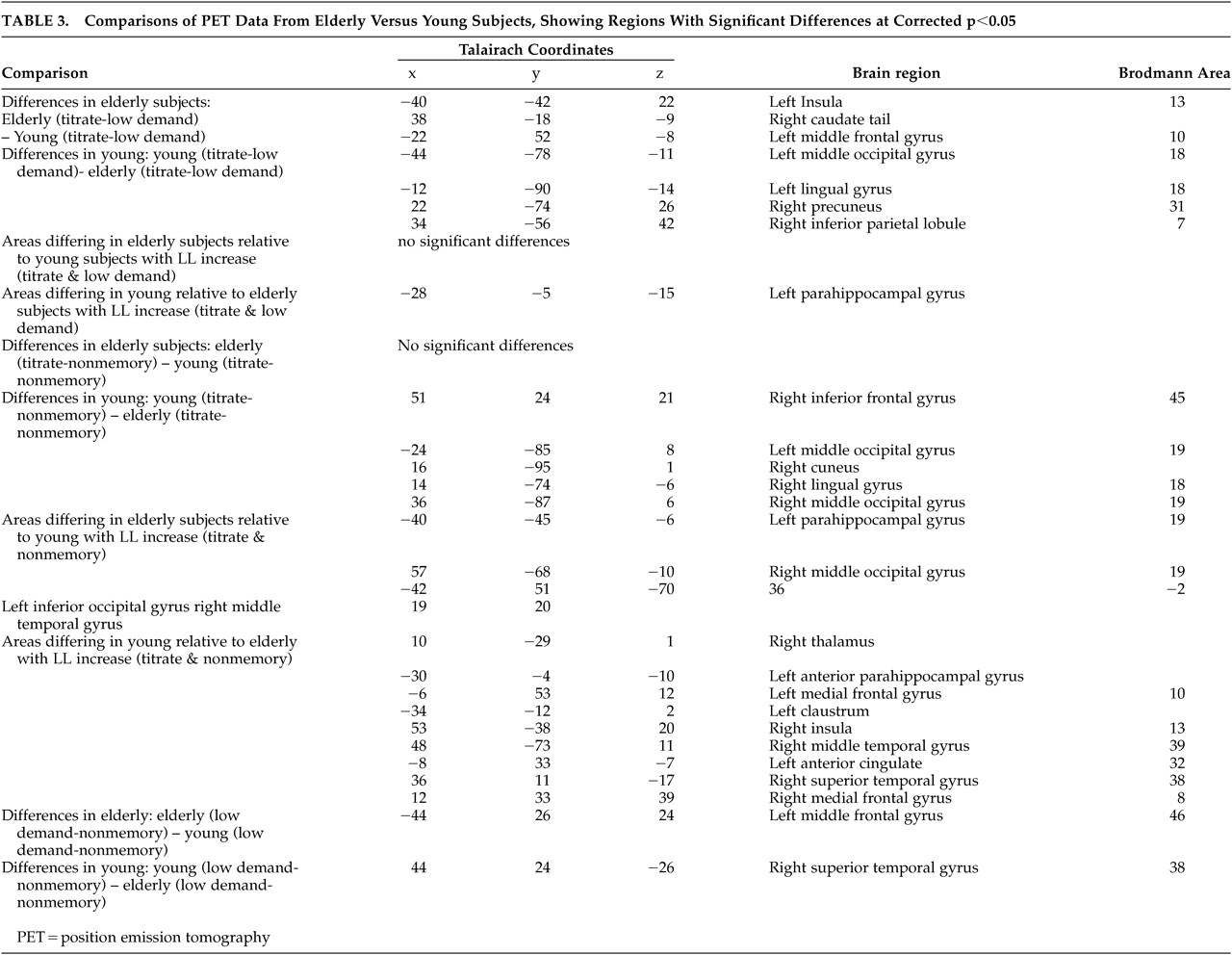
Young subjects attained a significantly longer study list size (SLS) than elderly subjects during nonverbal recognition. Between and within group differences in task difficulty were partially controlled for by titration. Even given this control for difficulty, residual differences in mean activation between the old and young subjects were seen for the Titrated demand task, compared with either one of the two controls. PET results showed, relative to young, elderly subjects engaged different regions, including the insula, during recognition, while young subjects engaged posterior brain regions. Regions where the two groups differed in the slope of Titrate minus control condition activation on SLS were also observed, with elderly subjects not showing the relationship seen in young between parahippocampal flow and SLS.
Our study differs from much of the work on aging and memory in that the Titrated demand memory condition attempted to match subjects on task difficulty. One critique of studies comparing different populations is that no attempt is made to match for differences between the groups, so that differences may be ascribed to amount of effort involved in task performance. In the current study, all subjects performed at approximately the same level of recognition accuracy. However, differences were still seen, with elderly subjects demonstrating greater differences in the bilateral insula and left middle frontal gyrus, among other regions. Young subjects showed greater mean activation in several regions, including bilateral posterior cortex. The overall recruitment of additional regions, relative to young subjects, by the elderly subjects during Titrate task performance was consistent with our hypothesis that age-related impairment would cause the elderly subjects to use alternate strategies in an attempt to maintain task performance. The decline in hippocampal changes, seen in elderly subjects relative to young, may reflect age-related impairment in this brain region.
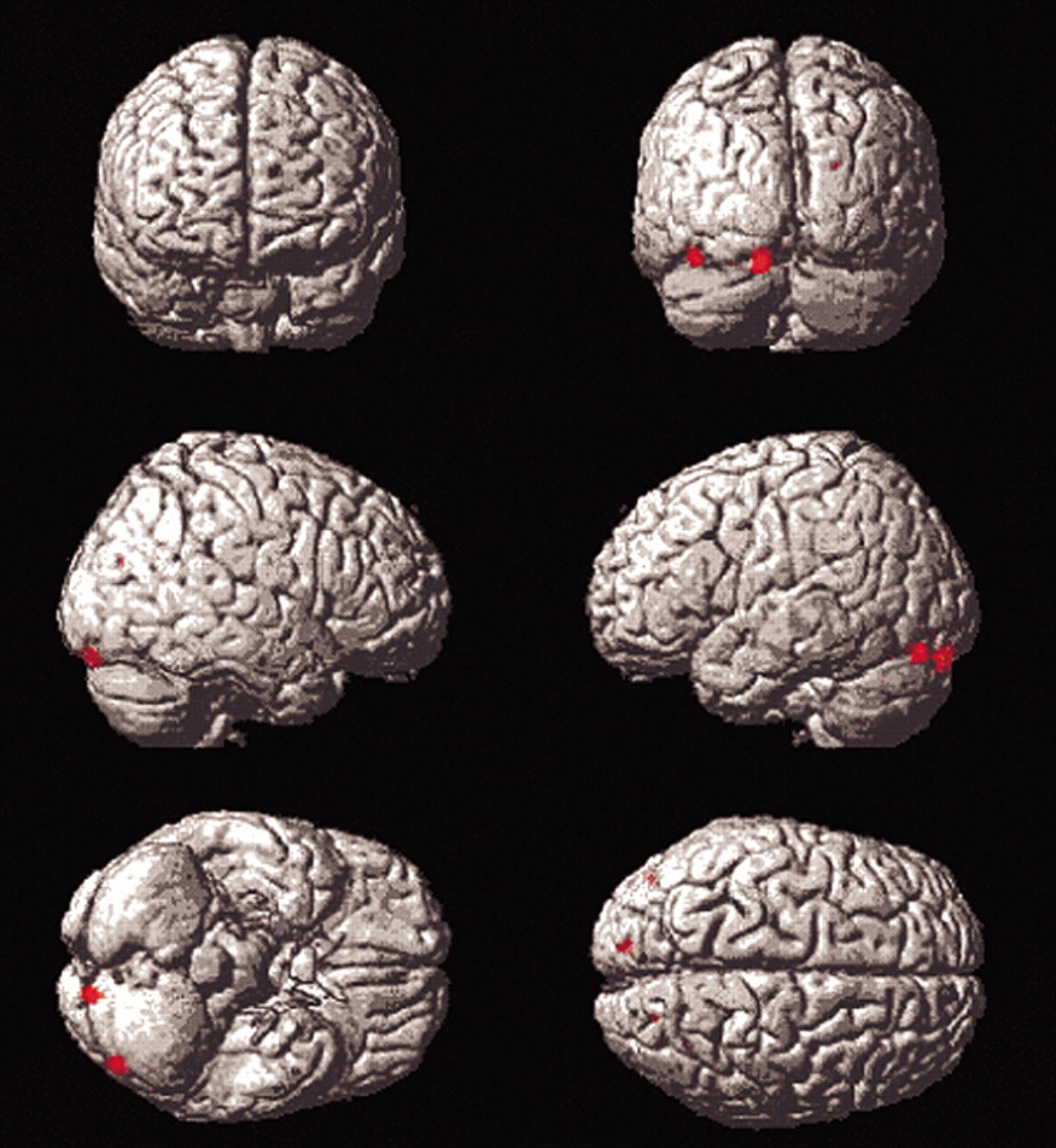
Differences in posterior blood flow seen in young subjects on direct comparison with elderly subjects on the Titrate task relative to the contitions of the two controls may reflect use of a different strategy by young subjects (
Figure 3). Occipital and temporal regions are part of a visual pathway involved in object perception
17 and encoding.
18 Prior work has found age-related decline in posterior blood flow during visual word identification.
10 Another PET study found that young subjects showed greater differences than elderly subjects in occipito-temporal and parietal areas during performance of verbal memory tasks, despite a lack of age-related effect on performance.
7 Thus, one of two differing strategies may be used, depending on age. Elderly subjects may no longer have access to posterior pathways, used by the young, due to age-associated neuronal changes. This may result in their utilization of alternate strategies, discussed below. Another explanation is that our differences in stimulus presentation times between the two groups, necessary to control for task difficulty, contributed to this effect in young subjects. Since young subjects spent more time viewing study stimuli at their faster presentation rate, they viewed more total stimuli during the Titrate condition.
Condition X group X SLS interactions for Titrate, compared with both control conditions were greater for the young than the elderly subjects in the left anterior parahippocampus. Primate work has shown that parahippocampal regions are engaged during recognition memory performance.
19,20 Human studies have shown the parahippocampal regions are crucial for encoding.
21 Early PET studies comparing young and elderly volunteers found reduced flow in the parahippocampal gyri at rest in elderly subjects, relative to young subjects, suggesting that function may decline with normal aging.
22In a functional magnetic resonance imaging (fMRI) study of young subjects, Witter and others
23 found, that anterior parahippocampal gyri differences occurred with object recognition. They suggested that the parahippocampal gyri coordinates bidirectional, topographically arranged transfer of information between higher cortical association areas and the hippocampus.
20,24 This suggests that the differences in modulation of frontal cortex seen in elderly subjects versus young volunteers in this study may be due, in part, to age-related parahippocampal changes.
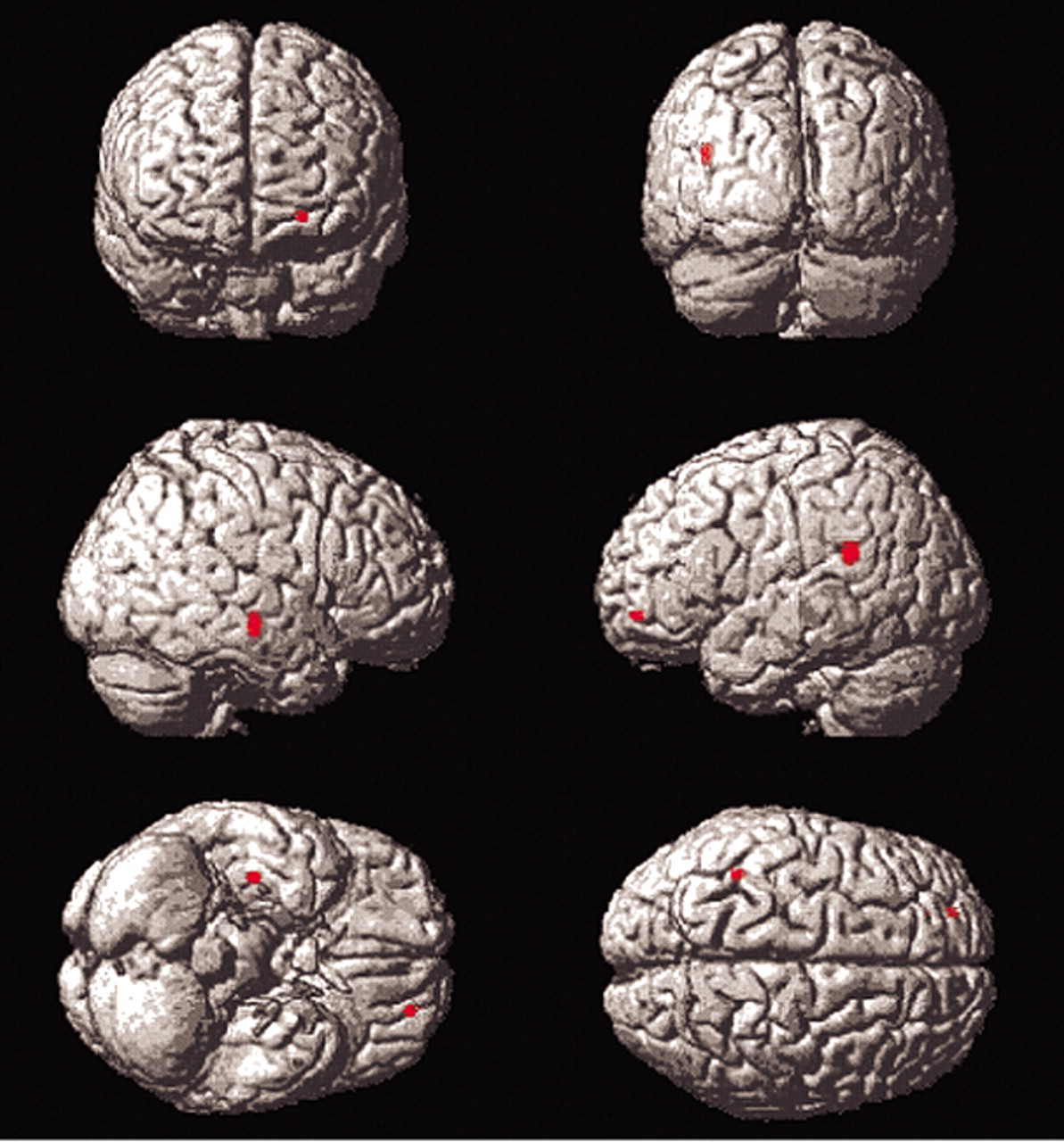
Insular differences were seen in elderly subjects relative to young subjects in the Titrate versus Low Demand comparison (
Figure 4). The insula is implicated in language and semantic memory retrieval (see Cabeza et al.). Young subjects’ suppression of the insula during difficult task performance may have contributed to their ability to attain significantly longer list lengths, since suppression of pathways through the insula may lead to improved episodic memory performance.
7 The differences in insular blood flow with increasing SLS on the correlational analyses of the Titrate versus Nonmemory control indicate that elderly subjects with higher SLS used the insula less. These findings are supported by the fact that elderly subjects showed insular differences on the Titrate versus Low Demand comparison and also demonstrated correlations with SLS in insula for the Titrate condition, compared to the Nonmemory condition. Thus, the elderly subjects showed insular differences with the more difficult task and modulated the insula differently than young subjects. An alternative interpretation is that the insular differences were related to covert naming by some subjects. The left insula has been implicated in object naming in other imaging work.
26,27 It may be that those subjects with poorer performance used a naming strategy, involving the insula.
On direct comparison, elderly subjects, relative to young, demonstrated greater mean differences in the left middle frontal gyrus on comparisons between Titrate and Low Demand and the two control conditions (areas 10 and 46, respectively,
Figure 4). Young subjects showed right-sided frontal differences, relative to elderly subjects, only on Titrate-Nonmemory (area 45) but not on the comparison of the two controls. This may be due to the fact that the Low Demand control poses more difficulty to elderly subjects than to young subjects, causing them to recruit areas used, in young, only for the more difficult tasks. Numerous other PET studies have reported differential difficulty-related frontal blood flow increases by young subjects, relative to elderly subjects. Jonides and others
8 found left lateral prefrontal cortex differences, when an
n-back memory task was made more difficult. The prefrontal cortex change with increased task challenge was not seen in elderly subjects, who also showed more interference during task performance. Similarly, Grady and others
28 in a study of face recognition found changes in prefrontal cortex blood flow (areas 9 and 46), as difficulty of face identification was increased by image degradation. Prior work by the same group
29 found that elderly subjects engaged regions associated with more difficult task performance in young subjects (such as prefrontal cortex) during performance of less difficult tasks. One alternative explanation that should be considered is that fatigue and motor learning effects on blood flow may have played a role since our tasks were not counterbalanced across conditions.
30Area 46 and the entire mid dorsolateral prefrontal cortex have been implicated in spatial memory, since lesions in this area cause selective spatial mnemonic impairment on spatial delayed response and alteration tasks.
31,32 Recent primate studies by Petrides
33 demonstrated a specific role for mid dorsolateral prefrontal cortex in monitoring information during tasks that require executive function. A PET study with young and elderly volunteers using delayed visual discrimination also found differences between young and old in brain regions engaged during task performance.
34 When networks connected to hippocampal differences were examined, they found that elderly subjects recruited a network including BA 9 and 46, and also middle cingulate and caudate, while young subjects engaged BA 10, fusiform, and posterior cingulate. Since groups were matched on performance, difficulty effects could not account for this finding. The authors suggest that large-scale network operations may change with normal aging, especially as hippocampal function declines. These frontal cortex findings in the current study may be indicative of the lack of hippocampal differences seen in elderly subjects, compared with young subjects, as task performance (SLS attained) increased.
In summary, this study demonstrates differences between elderly subjects and young subjects during performance of a nonverbal memory task. Relative to younger subjects, elderly subjects demonstrated blood flow changes in different regions, including the left insula, during performance of the titrated memory condition and failed to recruit prefrontal cortex. They also did not show the preponderance of occipital differences seen in young subjects on difficult tasks and failed to modulate parahippocampal blood flow, relative to the youngs, with increasing SLS. These differences may help delineate the locus of decline in processing abilities as an effect of normal aging.