Parkinson’s disease (PD) is a neurological condition affecting approximately 1% of people over age 60 in the U.S.
1 The neurological symptoms include slowness of movement, resting tremor, rigidity, and postural instability.
2,3 Psychiatric symptoms include the following: sleep disturbance, cognitive impairment, psychosis, anxiety, and depression.
4,5 Many people with PD suffer a decline in their physical functioning that adversely affects their quality of life.
6,7 Given the chronic and debilitating nature of PD, it is not surprising that many people suffer negative emotional consequences, particularly depression. This article examines the prevalence and treatment of depression in persons with PD.
We systematically reviewed the prevalence and treatment of depression in persons with PD using the Pubmed database. Our search was limited to English language articles. To identify articles on the prevalence of depression in persons with PD, we included all of the following keywords: Parkinson, depression, and prevalence. To identify articles on the treatment of depression, we used all the following keyword combinations: Parkinson’s and depression and treatment; Parkinson’s and selective serotonin reuptake inhibitors (SSRI); Parkinson’s and tricyclic antidepressants (TCA); Parkinson’s and antidepressants. These searches were augmented by bibliographical searches of the obtained references. In addition, we hand searched the following journals from 1999–2002: Movement Disorders, The Journal of Neuropsychiatry and Clinical Neurosciences, Psychosomatics, and Neurology.
Prevalence of Depression in PD
The actual rate of depression in persons with PD is unknown, but reported rates vary widely, from 7% to 76%.
6,8–22 Contributing to the width of this range is inconsistency in sampling procedures, assessment techniques, and definitions of depression.
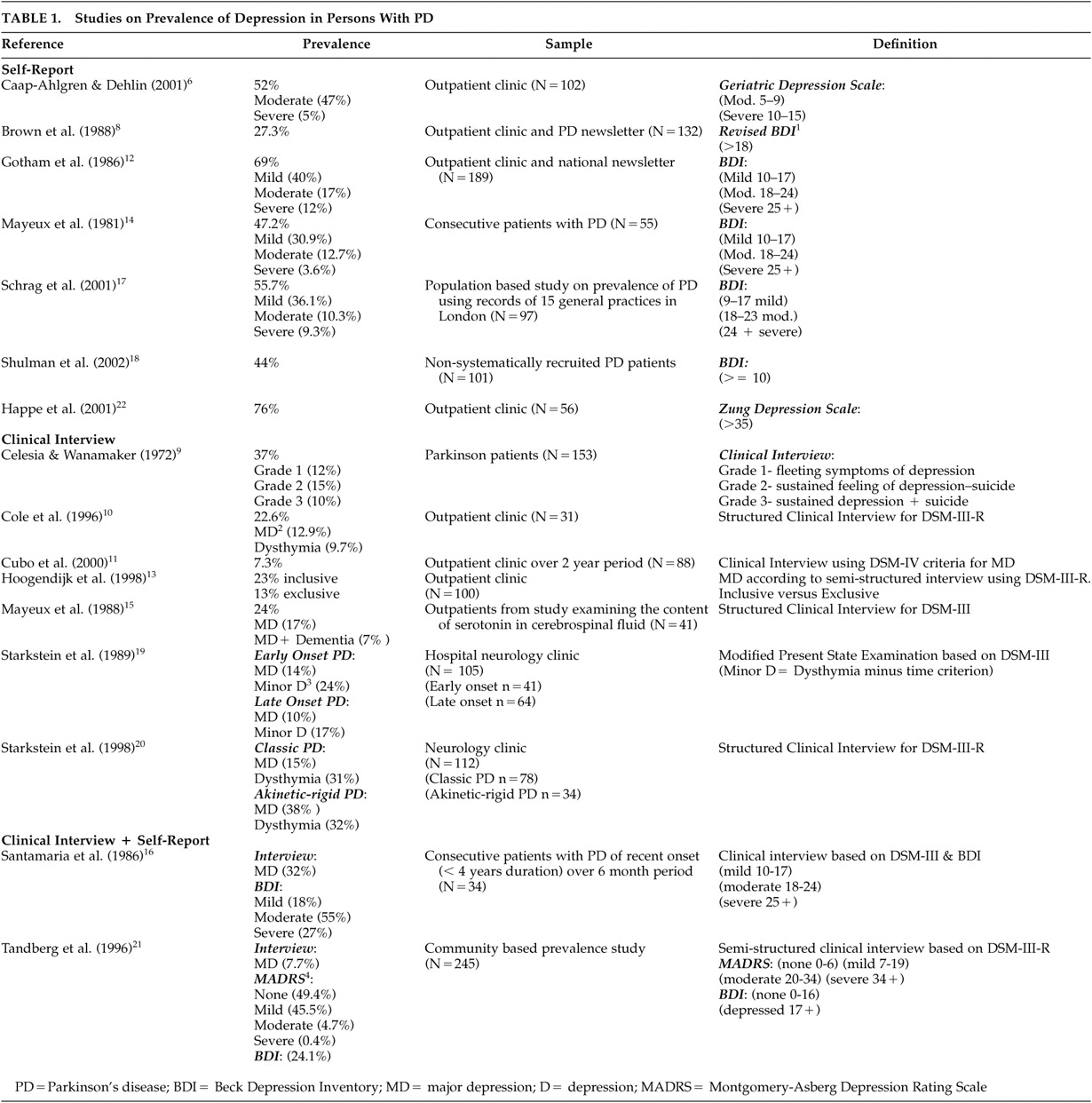
Table 1 summarizes the prevalence studies examined in this article.
Inconsistent Definitions of Depression in PD
The difficulty in defining depression in persons with PD is due, in part, to the overlap of symptoms of PD with those of depressive syndromes. For example, symptoms such as facial masking, in which facial muscles do not respond or move to reflect expression of emotions, give the appearance of a flat affect, which could be misinterpreted as evidence of depression. Slowness of movement due to PD could also be misinterpreted as a symptom of depression. Some researchers opt for an inclusive approach to diagnosing depression in PD, others for an exclusive one. In the former, the presence of a depressive symptom, regardless of origin, qualifies for the diagnosis of depression. In the exclusive method, symptoms thought to be due to PD are excluded from the diagnosis of depression. In a study by Hoogendijk et al. (1998)
13 100 PD patients were tested for major depression (MD), and prevalence was calculated using both exclusive and inclusive methods. In the exclusive method, symptoms were excluded if: (1) changes in the depressive symptom were associated with changes in PD signs and symptoms, or (2) the depressive symptom responded to dopaminergic medication. The inclusive approach excluded no symptoms in establishing the diagnosis of MD. The inclusive diagnostic method resulted in a prevalence of 23%; the exclusive approach resulted in a prevalence of 13%.
Regardless of origin, the symptoms of depression should be addressed and treated by physicians, and thus an inclusive approach is appropriate from a clinical standpoint. However, from a research standpoint, it is important to strive to determine the origin of depression in PD, and only through exclusive diagnoses is this possible. One such approach is to measure depression symptoms prior to treatment with PD medications and then measure the change in depression symptoms after PD medications have been administered. Consistencies in response to PD treatment could be examined across depression symptoms and thus allow some determination of when to treat depression symptoms as part of PD itself and when to treat depression symptoms as a separate entity.
Frequently, studies shared a common standardized instrument for assessing MD but used different cut scores in defining MD. This is evident in studies utilizing the Beck Depression Inventory (BDI). Of the studies using this instrument (N=7), only two utilized the same cut-score.
12,14 Gotham et al. (1986)
12 and Mayeux et al. (1981)
14 defined mild depression as a score between 10 and 17, moderate depression as a score between 18 and 24, and severe depression as a score of 25 or higher. Schrag et al. (2001)
17 closely duplicated these cut-score ranges of 9–17, 18–23, and 24 and above to define mild, moderate, and severe depression, respectively. Two other studies utilized the BDI. One defined depression as a score above 18;
8 the other defined significant depression as a score above 10.
18 Clearly, different methods of defining depression can substantially impact prevalence estimates.
Inconsistent Assessment Techniques
In the reviewed studies, the disparity in the reported rate of depression in PD can also be attributed to the use of different assessment techniques (e.g., the use of self-report questionnaires versus clinical interviews). In studies that utilized clinical interviews and defined depression based on the diagnostic criteria in the Diagnostic and Statistical Manual of Mental Disorders (DSM), Third Edition, Third Edition-Revised, and Fourth Edition, the prevalence estimates of major depression in PD ranged from 7.3% to 32%.
10,11,13,15,16,19–21 The reported rate of minor depression, which was defined as dysthymia in these studies, ranged from 9.7% to 31%.
10,19,20 When classification of depression was based on self-report questionnaires, estimated rates tended to be higher. They ranged from 27.3% to 69%, depending on how depression was defined.
6,8,12,14,17,18 This higher rate is thought to be due to symptom overlap, which is difficult to control for with self-report questionnaires.
Two studies utilized both clinical interviews based on DSM criteria and self-report questionnaires.
16,21 Santamaria et al. (1986)
16 reported a 32% rate of major depression based on DSM–III, and 27% based on a score of 25 or higher on the BDI. In contrast, Tandberg et al. (1996)
21 reported a rate of 7.7% of major depression based on DSM–III–R criteria, and 25.1% based on a score of 17 or higher on the BDI. The disparity in these prevalence estimates highlights the need for utilization of consistent measures of depression by researchers examining depression in PD.
Inconsistent Sampling Methods
Another problem contributing to the range in the reported rates of depression was the use of varied sampling methods. Only two studies were community-based prevalence studies.
17,21 In these studies, the reported rate of moderate-to-severe depression was 19.6% (BDI >= 18)
17 and 24.1% (BDI >= 17)
21 based on self-report questionnaires, and 7.7% based on clinical interviews utilizing DSM–III–R criteria.
21 The remainder of the studies recruited patients from outpatient neurology clinics, and the rates in these studies ranged from 7.3% to 53% for both major and minor depression as defined by DSM criteria
10,11,13,15,16,19–21 and 27.3% to 69% based on self-report questionnaires.
6,8,12,14,17,18 The higher rates observed in outpatient neurology clinics are consistent with research findings indicating higher rates of depression among older adults seen in primary health care clinics compared to those seen in community settings.
23Inconsistencies in methods used for studying depression in PD have led to a wide range of reported prevalence, from 7% to 52%. In a recent review, Slaughter et al.
24 suggested that the true rate of depression in PD patients is around 31%, based on a combination of findings from 45 studies. However, the varying methodologies of the studies were not taken into account in this report. It is suspected that if community based prevalence studies were conducted, the “true” rate would be somewhat lower. Despite these problems with the diagnosis and classification of depression in PD, most studies found it to be a relatively common problem among persons with PD.
Etiology of Depression in PD
There is debate regarding the cause of depression in PD patients. Depression in PD could be exogenous, a reaction to being diagnosed with a disabling, chronic illness for which there is no known cure. Some researchers, however, believe that depression in PD may be endogenous and caused by neurological changes associated with the disease process itself. Research examining the theory that depression is exogenous has evaluated the impact of a number of variables on depression. These include disease severity, disease duration, age of onset, and gender. No consistent relationship has been found between these variables and depression.
25 A study examining changes in disability and depression suggested that depression may be associated with more rapidly progressive PD.
26 Further, a population-based study examining this issue found depression in PD to be predicted by impaired cognitive functioning and the presence of a thought disorder, suggesting that the depression was a primary consequence of brain dysfunction.
27 There is a biochemical basis for the hypothesis that the depression observed in PD is mainly the result of neurodegeneration. The etiology of PD involves changes in three neurotransmitter pathways: D
2, noradrenaline, and serotonin (5–HT). Serotonergic pathways have been implicated in depression in general and in depression associated with PD, in particular.
1 If depression is exogenously determined in PD, its prevalence among PD patients should compare to its prevalence in patients with other chronic disabling diseases. Studies comparing the prevalence of depression in PD and in other chronic debilitating diseases have found no consistent differences in patterns.
26 Finally, in some cases of depression in PD, depression may be tied to the treatment of PD itself. Mood swings accompany late stage fluctuations in response to
L-dopa, known as the “on-off” phenomena. As many as two thirds of patients with PD experience “on-off” symptoms. Some patients fulfill the clinical criteria for major depression during the “off” periods.
28It is likely that the relationship between PD and depression is complex and that a single explanation cannot account for the majority of the observed cases of depression. The two main theories (that depression is exogenous or that it is endogenous) are not mutually incompatible.
25 The finding that higher frequencies of depression occur in the early and late stages of PD
1,26 suggests that depression is due to a combination of both the subjective reaction to the disease and the underlying brain dysfunctions caused by the disease process.
Adopting a biopsychosocial view of depression in PD necessitates the development of effective treatments addressing both the underlying neurological dysfunction as well as the exogenous factors contributing to depression in persons with PD. The following section reviews the evidence for the treatment of depression in PD, which, unfortunately, is sparse.
Treatment of Depression in PD
There are many and varied methods for the treatment of depression. Psychotherapy, antidepressant medication, and ECT have all been shown to be viable treatment options for depression.
2,26,29–31 Despite the wealth of research regarding the treatment of depression, there is limited information specific to its treatment in persons with PD.
Despite this lack of empirical evidence regarding the treatment of depression in persons with PD, several authors have put forth treatment algorithms reflecting current best practice guidelines for treating depression in persons with PD.
2,32,33 These algorithms include three common suggestions. One suggestion is to first treat the person’s PD symptoms optimally with antiparkinsonian medications before attempting to treat depression.
33 Once optimal PD treatment has been achieved, treatment aimed specifically at depression should be implemented. Another suggestion is the utilization of an antidepressant medication.
2,32 SSRIs and TCAs represent the most frequently selected classes of antidepressants. Another suggestion is psychosocial support and behavioral therapy to minimize stress and increase the quality of life of persons who have PD and depression. Electroconvulsive therapy is currently viewed as a treatment of last resort.
2,32 These suggestions reflect current best clinical practice and have not been tested rigorously in PD populations. The empirical evidence is now reviewed for each of the following components: medication therapy, ECT, psychotherapy, and other treatment options. There is often limited evidence to guide the application of these treatments. However, recommendations made reflect current best practice guidelines.
Medication Therapy for Depression in PD
One concern with the administration of medications for treatment of depression in PD patients is potential for adverse neurological side effects, including motor effects, hallucinations, and delusions, as well as interactions between the medications used to treat PD and antidepressants, which could produce mental changes (e.g., delusions, confusion) and other symptoms.
26Dopamine Replenishing Strategies
The finding that dopamine (D
2)-replenishing strategies used to control motor symptoms of PD may also improve depression in persons with PD
34,35 is controversial. Some research has demonstrated improvement in depression in Parkinson’s disease patients after the administration of
L-dopa treatment,
9,36 but other research failed to confirm this finding.
15,25,37 It has even been suggested that
L-dopa may produce depression.
38 L-dopa therapy is an unlikely cause of depression, since frequency of depression in persons with PD is similar before and after treatment with
L-dopa.
37The role of D
2 agonists in the treatment of depression is still being explored. There is a lack of well-designed controlled studies in this area, but preliminary research suggests that bromocriptine and pramipexole may have mood-altering effects.
1,37,39 Dopamine substitution may be effective in the treatment of depression in persons with PD, and from a clinical standpoint it is important to treat patients optimally for their parkinsonian symptoms before attempting to treat depression. This is especially salient for PD patients showing on-off motor fluctuations, as patients may exhibit depressive symptoms as a consequence of “off” periods.
Other Anti-Parkinsonian Drugs
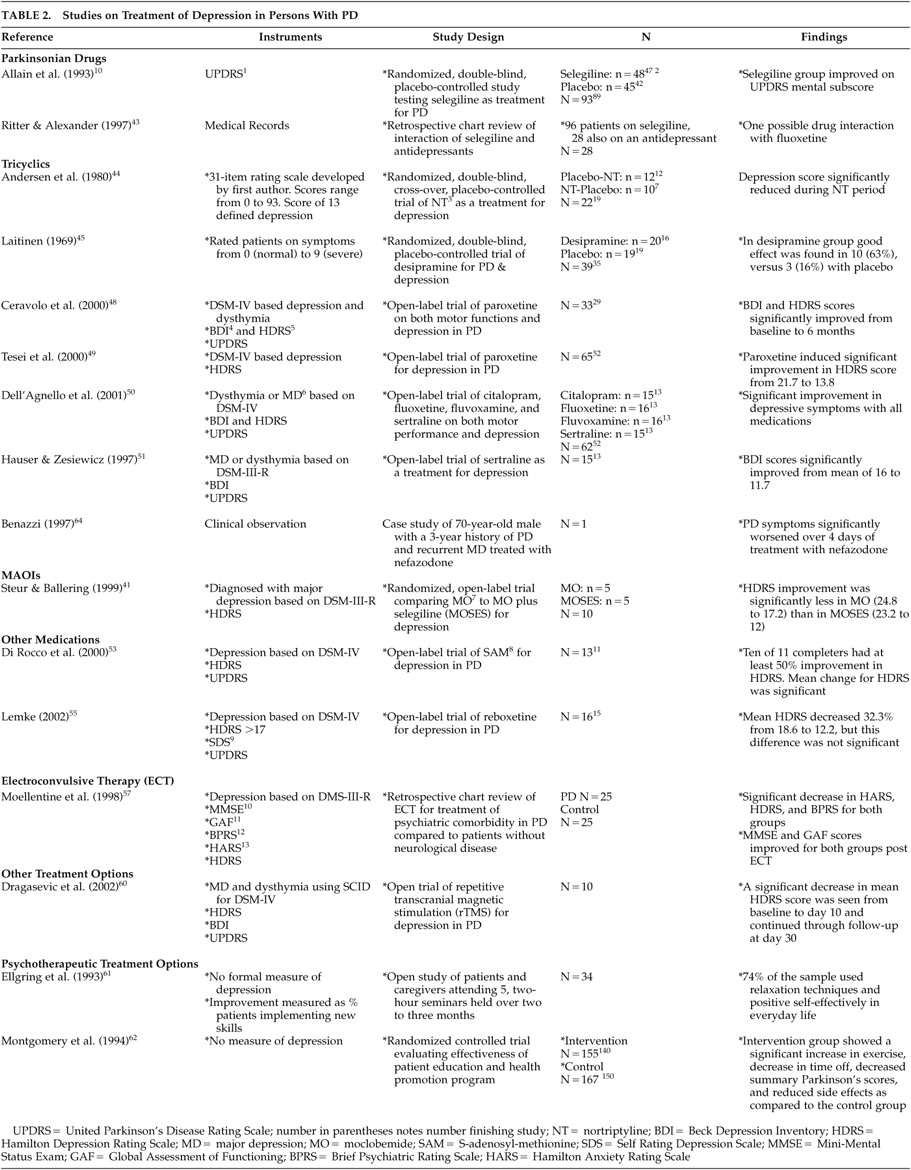
Selegiline is a monoamine oxidase B (MAO-B) inhibitor that has been shown to be effective as a treatment for PD. At least two studies demonstrated selegiline’s effectiveness for treating depressed mood in persons with PD.
40,41 The major complication with the use of selegiline in depressed persons with PD is its potential for a fatal effect when combined with certain SSRIs. This serotonin syndrome is characterized by mental status changes, tremor, myoclonus, diarrhea, hyperpyrexia, hyperreflexia, and diaphoresis.
42 Although the reported incidence of serious reactions is very low,
42 the United States Food and Drug Administration recommends against the use of selegiline and certain antidepressants simultaneously. In a retrospective chart review of 28 PD patients taking both selegiline and an antidepressant,
43 only one report of an adverse reaction of selegiline combined with fluoxetine was identified.
The beneficial effect of selegiline on mood was discovered during its test as a treatment for PD itself.
40 In this study, patients were actually excluded on the basis of depression by a Hamilton Depression Rating Scale (HDRS) >25. However, patients placed on selegiline showed an improvement in mood on the United Parkinson’s Disease Rating Scale (UPDRS) mental component compared to patients placed on placebo. In a second randomized study, comparing moclobemide to moclobemide plus selegiline,
41 selegiline was shown to enhance the effectiveness of moclobemide alone in lowering depression symptoms in PD patients. Negligible adverse side effects due to selegiline treatment were reported but were reversible upon discontinuation of the drug.
Thus, while there is concern for the possible adverse effects of combining selegiline with certain antidepressants, its clinical significance is not well substantiated. The one study showing selegiline to enhance the effects of an MAO inhibitor is promising, but more controlled research is needed to determine the effectiveness of selegiline alone as treatment for depression in persons with PD as well as its possible role as an augmenter of certain antidepressants.
Tricyclic Antidepressants
The tricyclic antidepressants inhibit the reuptake of serotonin and norepinephrine and produce long-term increases in sensitivity in these receptors. The TCAs include the following: amitriptyline, nortriptyline, imipramine, desipramine, and doxepine. These medications vary in their abilities to block the reuptake of norepinephrine and serotonin. They also vary in their plasma half-lives and clearances and have different antidepressant, anticholinergic, and sedative properties. The sedative and anticholinergic properties may make them beneficial in the treatment of tremor. Despite this benefit, the risk from anticholinergic side effects of the TCAs is very high in this population. Such side effects include dry mouth, blurred vision, urinary retention, paralytic ileus, precipitation of acute glycoma, tremor, cognitive symptoms, memory impairment, and confusional states, possibly even delirium, especially at higher doses. When patients are already taking an anticholinergic medication, a common occurrence in this population, TCAs are contraindicated. Furthermore, all TCAs can slow cardiac conduction, and they should be used carefully in persons with coexisting cardiac problems. Additional adverse side effects include the increased risk of falls due to orthostatic hypotension and the stimulation of the histamine receptors, which may cause sedation and weight gain.
There is little research on the use of these medications in persons with PD. Two double blind studies reported on the effectiveness of nortriptyline and desipramine in PD patients with depression.
44,45 It should be noted that both of these studies were conducted over 20 years ago, and both utilized measures of depression that were not well validated. Nortriptyline was compared to placebo in a double blind crossover study of 22 patients from two outpatient clinics.
44 Crossover periods were at 8 weeks, and the entire trial lasted 16 weeks. Depression, as measured by a 31-item rating scale, was reduced during the nortriptyline period as compared to the placebo period. The only adverse effect was the significant lowering of systolic blood pressure in two patients who were discontinued from the trial. The second study
45 compared desipramine to placebo in a double-blind fashion, with 20 patients receiving the drug and 19 receiving placebo. Depression was rated by the authors from 0 (normal) to 9 (maximal severity). Desipramine was titrated to 100 mg over 1 week, and the entire trial lasted 3 weeks. Improvement in depression was reported in nine patients in the desipramine group.
In this population TCAs must be used carefully, and patients must be monitored closely for the possibility of adverse side effects. In persons with PD, it is best to use medications with a short plasma half-life, a rapid clearance, and little anticholinergic activity.
29 Desipramine and nortriptyline have the least anticholinergic activity and the least sedative properties and thus may be preferred in PD patients with cognitive impairment. Tricyclic antidepressants that should be avoided in persons with PD include amitriptyline, due to its sedating properties; amoxepine, due to its D
2 blocking properties; and doxepine, due to its PD-like adverse reactions. Practically speaking, TCAs are most beneficial to PD patients with prominent anxiety and sleep disorders. Due to their high side effect profile, TCAs are often contraindicated in older adult populations with neurodegenerative diseases other than PD. However, due to the beneficial effects of their anticholinergic properties on parkinsonian symptoms as well as depression, TCAs may be less contraindicated in PD patients.
SSRIs
The SSRIs represent the most commonly used medications in the treatment of depression in persons with PD. A recent survey by the Parkinson’s Study Group found that SSRIs are used as a first line treatment of depression 51% of the time, with TCAs being used 41% of the time, and other agents 8% of the time.
1 SSRIs are often chosen as a treatment of depression in persons with PD due to their low side effect profile.
Selective serotonin reuptake inhibitors have been found to be well tolerated in populations of elderly depressed patients. Specifically, they are related to lower incidences of anticholinergic side effects and cardiac adverse events.
29 Unlike the TCAs, the SSRIs stimulate rather than sedate, which may be preferable in apathetic, anergic, passive, and withdrawn patients. Common adverse reactions with the SSRIs include gastrointestinal complaints and sexual dysfunction. Selective serotonin reuptake inhibitors have also been reported to cause patients to develop movement disorders, either de novo or as an exacerbation of an underlying condition such as PD. Improvement
46 and worsening of parkinsonian motor problems during SSRI treatment of depression in PD have both been reported.
47 As mentioned previously, a notable contraindication for the use of SSRIs in the treatment of depression in persons with PD is their adverse reaction with selegiline.
Despite the preference for utilizing these drugs as the mainstay of treatment for depression in persons with PD, there are no adequately controlled clinical trials of the use of these medications to treat depression in persons with PD. Paroxetine has been shown to improve depressive symptoms in two studies.
48,49 In the first study, 33 patients with PD and depression were subjected to an open trial of 20 mg of paroxetine over a period of 6 months. Four patients dropped out of the studies; two (6%) due to visual hallucinations. Clear improvement in mood on the BDI and HDRS was shown in 25 of the 29 completers. Only one (3%) participant showed a clear worsening of PD tremor, which returned to normal after paroxetine was removed. In the second study,
49 paroxetine’s tolerability was examined in 65 PD patients with major depression. Paroxetine was titrated to 20 mg over 4 weeks, and the entire trial lasted 3 months. Fifty-two participants completed the study, with 13 (20%) dropping out due to adverse effects, including anxiety (N=4; 6%), nausea (N=4; 6%), increased “off” time duration and exacerbation of parkinsonian tremor (N=2; 3.5%), agitation (N=1; 1.5%), confusion (N=1; 1.5%), and headache (N=1; 1.5%). There was significant improvement in depression, as measured by the HDRS, during the trial. Thus, paroxetine has been shown to improve depressed mood in PD patients with depression in two open-label trials. However, PD symptoms were worsened in a number of participants. All side effects proved reversible with removal of the drug.
Sertraline has been shown to improve BDI and HDRS scores in two open-label trials
50,51 with a total of 30 patients. In both studies, side effects were minimal and included lightheadedness, insomnia, ankle edema, and increased irritability. All were reversible upon removal of the drug.
Citalopram, fluoxetine, fluvoxamine, and sertraline were studied in a single open label trial in 62 patients with PD and depression.
50 Significant improvement in depression, as measured by the BDI and HDRS, was shown from baseline to 6 months for all medications, with no differential effectiveness for any of the drugs. On average, improvement in mood started after 19 days of treatment. Side effects were minimal and included increased irritability and excessive sedation, and these effects were reversible upon discontinuation of the medications.
The SSRIs are the most commonly chosen medications for the treatment of depression in persons with PD. Their advantages outweigh their potential adverse effects. One major finding from the studies reviewed is that when PD symptoms worsened with the use of SSRIs, this worsening only occurred in a small percentage of patients and was reversible upon removal of the medication. Randomized controlled trials are now needed to demonstrate the true efficacy of SSRIs in this population.
Other Medication Treatment Options
Several other classes of medications have been studied to evaluate their effectiveness in the treatment of depression in persons with PD. These medications include S-Adenosyl-Methionine (SAM) and reboxetine.
S-Adenosyl-Methionine is a newer alternative treatment for depression. It is a methyl donor in the brain, involved in the pathways for the synthesis of hormones, neurotransmitters, nucleic acids, proteins, and phospholipids. It is hypothesized to have its antidepressant effect due to its involvement in the synthesis of norepinephrine, D
2, and serotonin.
52 In a recent review of the literature on SAM,
52 it was found to be superior to placebo and as effective as the TCAs in the treatment of depression at doses between 200 and 1600 mg/d. It is also well tolerated, and side effects are minimal and include mild insomnia, lack of appetite, constipation, nausea, dry mouth, sweating, dizziness, and nervousness. However, there is little information on the possible drug interaction effects of SAM.
S-Adenosyl-Methionine was studied as a treatment option for depression in 13 patients with PD who had not responded to previous antidepressant medications in an open label trial.
53 S-Adenosyl-Methionine was titrated to 3600 mg a day for a period of 6 weeks, and the trial continued for 10 weeks. Two (15%) patients dropped out of the trial due to intolerable anxiety. There was a significant decrease in HDRS scores for completers, with 10 participants showing a 50% decrease in symptoms and only one participant not responding to SAM. No patient reported a worsening of PD symptoms during the trial.
Reboxetine is a selective noradrenaline reuptake inhibitor, hypothesized to have its antidepressant effect by increasing the availability of noradrenaline in the central nervous system (CNS). Reboxetine is the first purely selective noradrenaline reuptake inhibitor exhibiting little or no action on serotonergic transmission and negligible affinity for other receptors in the brain.
54 Reboxetine was tested in an open label trial of 16 patients with PD and depression.
55 The drug was titrated to a mean of 6.13 mg a day, and treatment lasted for 28 days. One (6%) patient dropped out due to the development of delusions and visual hallucinations, and two (12.5%) patients showed a significant increase in hand tremor. There was a significant improvement in HDRS scores for the 15 completers.
ECT
Electroconvulsive therapy has been reported for treatment of both depression in PD and for symptoms of PD itself. While the precise mechanism of action of ECT on both depression and PD symptoms is not known, it is hypothesized that ECT achieves its beneficial effects through the stimulation of various neurotransmitter systems, including D
2. There have been numerous case studies reporting on the value of ECT for treatment of depression in PD, but there are no controlled trials. Further, the (
Table 1) with ECT have not included objective measures of either depression or PD symptoms. Two articles are included in this section; one is a systematic review of the literature on ECT as a treatment for depression in PD that reports on 27 studies conducted from 1975 to 1990.
56 A more recent retrospective study reports on the routine use of ECT in 25 patients.
57 Of the 27 studies reported in the review,
56 only one was a controlled study.
58 It examined ECT as a treatment for PD in a sample of 11 participants without psychiatric comorbidity. Of the 27 studies, 20 examined comorbid depression and PD. However, none of these included more than 7 participants, and the majority (N=13) were single case reports. There was no adequate report of objective measures of depression in these studies. The review reported that approximately 50% of patients receiving ECT, regardless of the presence or absence of psychiatric comorbidity, showed improvement in parkinsonian symptoms.
56A more recent chart review of all patients receiving ECT at a large teaching hospital over a 32-month period found 25 patients with PD received ECT for psychiatric comorbidity. These included major depression with and without psychosis, anxiety disorder (not otherwise specified), and dementia. Results were matched against 25 comparison subjects who received ECT for psychiatric conditions but did not have a comorbid neurological condition.
57 The study found that ECT improved depression, anxiety, cognitive status, and overall global functioning in both groups. There was also a subjective report of an improvement in Parkinson’s symptoms in 56% of the Parkinson’s disease patients. However, the Parkinson’s group experienced more complications as a result of ECT (56%) compared to the comparison group (12%). The most frequent complication experienced was transient intertreatment delirium necessitating the postponement or termination of treatment. Fifty-two percent of the PD group experienced such a delirium, compared to 20% of the comparison group patients. Other complications for the PD group included urinary retention, choreiform movements, and falling.
Thus, the effectiveness of ECT for use specifically in PD has not been submitted to rigorous scientific scrutiny. From the published review and the recent study described above, it appears that ECT can be effective in alleviating psychiatric symptoms and have at least short-term benefit for patients with parkinsonian symptoms. These gains are not without complications, which should be carefully weighed against the benefits of ECT. Given the long-term benefits of ECT appear to be largely unknown, its use in the treatment for depression in PD patients should be viewed with caution.
Other Treatment Options
Repetitive transcranial magnetic stimulation (rTMS) has been touted as a safer alternative to ECT for use in treating depression. It is being explored as a possible treatment for PD symptoms, as well as for the treatment of depression in PD patients. Unlike conventional ECT, rTMS administers electric stimuli to a highly localized region of the brain. Repetitive transcranial magnetic stimulation uses less voltage than ECT and does not have the side effects associated with ECT.
59 The positive effects of rTMS on the motor symptoms of PD have been shown to persist for 3 months following treatment.
59 In an open trial with a sample of 10 patients,
60 a series of rTMS over 10 days improved depression for up to 20 days following treatment.
Psychotherapeutic Treatment Options
Given the possibility of adverse interactions between antidepressant medications and antiparkinsonian medications, nonpharmaceutical treatment options for depression in PD patients are highly desirable. Psychotherapeutic treatment options, which have proven effective in treating depression in chronically medically ill populations, are especially worth considering. There have been two published articles
61,62 examining the usefulness of psychotherapeutic techniques and patient education for improving negative emotions and other health outcomes in persons with PD.
In the first study, Ellgring et al.
61 examined the usefulness of Cognitive Behavioral Techniques including stress management, cognitive restructuring, social skills training, modeling, role playing, and relaxation training. Skills were taught to both patients and caregivers in a series of five 2-hour seminars held over the course of two to 3 months. No measures of depression were administered. Outcome was determined as percent of patients implementing the skills in their daily lives. Seventy-four percent of the sample used relaxation techniques and positive self-instruction in their everyday life. The authors determined that group size was related to outcome, with those attending groups of five or six people using strategies more often than those attending groups of nine participants.
In the only randomized, controlled trial of a psychotherapeutic treatment option in a PD patient population, Montgomery et al.
62 examined the usefulness of an intervention delivered via the mail. The intervention included administration of a disease assessment questionnaire completed by the patient at 0, 2, 4, and 6 months. Both participants and participants’ physicians received feedback based on responses to this questionnaire. Patients received feedback, including exercise recommendations, disease severity, diet, compliance, side effect control, and information about dealing with specific reported problems. The physician received a summary of the patient’s responses and additional feedback, including suggestions to consider adjusting medication dosage based on the patient’s report of side effects. Again, there was no formal measure of depression. When matched against the comparison group, patients in the intervention group reported a significant increase in exercise, decreased “time off,” reduced side effects, and a decreased summary score from the Unified Parkinson’s Disease Rating Scale, which is an average of on-score, off-score, side effects index, and the patient’s global assessment. Other improvements for the intervention group included decreases in
L-dopa dose and in the number of doctor visits, hospital days, and sick days. In addition patient self-efficacy scores, measured by a battery of 15 questions developed for this study, improved.
While these studies suggest that nonpharmaceutical treatment options may improve quality of life and health outcomes in PD patients, neither focused specifically on improving psychiatric comorbidity. Given the effectiveness of techniques such as cognitive behavioral therapy (CBT) in other chronically medically ill populations,
63 the lack of research specific to the psychotherapeutic treatment of depression in PD patients is surprising.
CONCLUSIONS
This review of the literature on depression in PD points to several areas where knowledge and research are lacking. The review of studies on the prevalence of depression in the population points to the need for consistent use of well-validated definitions and measures of depression when obtaining prevalence estimates. Inconsistency in definitions, assessment techniques, and sampling methods has resulted in a wide range of prevalence estimates and uncertainty regarding the rate of depression in persons with PD.
Research efforts focusing on determining the prevalence of depression in persons with PD should strive for standardized sampling and assessment techniques. Research aimed at assessing “true” prevalence estimates should focus on large community based sampling methods. However, research aimed at addressing the prevalence of depression in PD patients attending outpatient health clinics should focus on randomized samples of PD patients in such settings.
A two-tiered approach aimed at identifying patients in outpatient settings in need of treatment for depression has been found to be useful in other outpatient settings.
66 Such an approach would include first screening patients for depression utilizing brief instruments, such as the two-item depression screener from the Prime-MD,
66 and then conducting a more comprehensive assessment of the patient’s psychiatric functioning.
Once patients have screened positive for depression, they should undergo a multidimensional assessment of biological symptoms, cognitive symptoms, affective symptoms, and overt behaviors which would be useful for helping researchers, as well as clinicians, rate the severity of depressive symptoms along each of these dimensions. Such a comprehensive assessment would include self-report instruments such as the Patient Health Questionnaire-9, which has been shown to adequately detect depression in other outpatient medical settings.
65 Further, research protocols should include a structured clinical interview, such as the Structured Clinical Interview for DSM–IV Axis-I Disorders,
67 assessing other psychiatric diagnoses, which need to be addressed. An assessment of the patient’s quality of life and functioning in areas such as work, recreational activities, and relationships should also be included. The Parkinson’s Disease Questionnaire-39 represents
68 such a measure of quality of life in PD. Finally, a screening of the person’s cognitive functioning, such as the Mini-Mental State Examination,
69 is warranted given the occurrence of dementia in this population.
Some argue the importance of distinguishing depressive symptoms due to neurological changes brought about by PD from depressive symptoms due to processes separate from PD. There is little to guide this distinction. Regardless of origin the symptoms of depression should be addressed and treated by physicians in routine clinical practice. However, from a research standpoint, it is important to continue research efforts aimed at distinguishing the origin of depressive symptoms in PD.
The research examining treatment of depression in persons with PD indicates that SSRIs are the most frequently utilized pharmacological treatment for depression. However, none of the studies that evaluated SSRIs were randomized controlled trials. There are two randomized controlled trials that support the use of TCAs, but the studies were conducted over 20 years ago without well-validated measures of depression. In addition to standard antidepressants, other “alternative” treatment options are being explored and include SAM, reboxetine, ECT, and rTMS. All of these methods show promise as possible treatment options. However, these treatment options have not been subjected to more scientific scrutiny. Finally, psychotherapy treatment options for depression could be highly desirable in persons with PD, given concerns regarding medication interaction effects. However, viable psychotherapeutic treatment options specifically targeting depression have not been studied.
There is a great need for systematic and controlled research examining both the prevalence and treatment of depression in persons with PD. Based on the literature and current clinical practice, the most promising option may be treatment with SSRIs. The possibility of adverse effects arising from administering SSRIs and selegiline in combination, however, warrants exploration of nonpharmaceutical options, such as psychotherapy. Although many articles point to the usefulness of psychotherapy for treating depression, to our knowledge, there is not one published controlled trial evaluating the effects of psychotherapy in persons with PD.
ACKNOWLEDGMENTS
This study was supported (or supported in part) by the Health Services Research and Development Service, the Office of Research and Development, and the Department of Veterans Affairs.