Throughout early fetal life, the face and anterior brain evolve in exquisite embryological intimacy.
1–3 This unity is responsible for facial dysmorphogenesis in disorders of early brain development with their associated cognitive and other deficits in adulthood.
4,5 These disorders range from major chromosomal abnormalities such as Down’s syndrome to less readily recognizable conditions such as velocardiofacial syndrome. In a complementary manner, individuals with facial dysmorphogenesis such as cleft lip/palate have been reported recently to show abnormalities of brain structure
6 in association with cognitive deficits.
7Recently, the application of anthropometric techniques has indicated that subtle facial dysmorphology is present also in schizophrenia.
8–10 Such findings may be an important biological counterpart of epidemiological and other evidence that early developmental disturbances are involved in the origins of this disorder.
9,11,12 It has also been reported that facial dysmorphology accompanies abnormalities of brain structure in a putative nonhuman primate model of schizophrenia involving early fetal insult.
13 However, realization of the full potential of facial dysmorphology for informing incisively on the developmental biology of schizophrenia is predicated on clarification of a critical issue: in addition to its embryological relationship to brain structure, to what extent can it be shown that variation in facial morphology reflects variation in adult brain function? To address this challenge requires a technique for resolving and quantifying the detailed topography of facial [dys]morphology in a manner that allows it to be related to an index of brain function such as cognition.
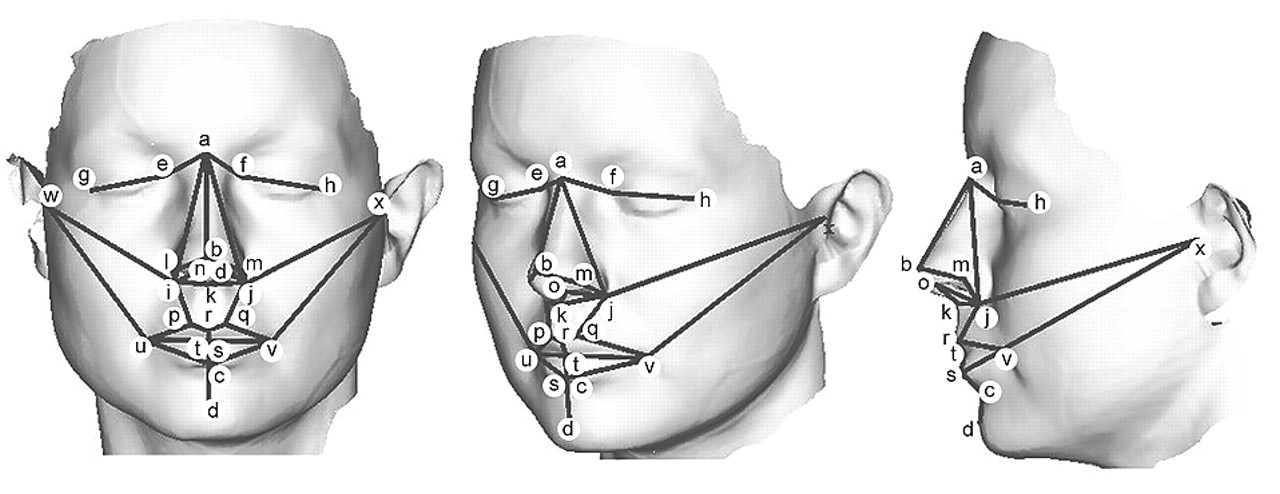
As facial development is an intrinsically three-dimensional process, efforts to index facial shape with conventional approaches such as linear anthropometry fail to take into account fundamental geometric aspects of this process. As an interim approach, the authors have recently obtained useful additional information by comparing patients with schizophrenia and healthy subjects using three-dimensional facial configurations reconstructed from linear anthropometric measurements.
14 However, three-dimensional digitization technologies now allow facial surfaces to be recorded and landmark coordinates obtained, while geometric morphometrics, which analyze three-dimensional landmark coordinates directly, provides the basis for relating shape to other biological variables both statistically and visually.
14,15 Portable, hand-held three-dimensional laser surface scanning has become available, and the authors have recently applied this technique to specify statistically and anatomically the nature of sex differences in facial morphogenesis in human subjects.
16 In the present study the authors have examined the relationship between cognitive ability and facial shape in a normal population, as captured using three-dimensional laser surface scanning, to establish a relationship between facial morphogenesis and adult brain function as a basis for conducting subsequent studies in schizophrenia.
DISCUSSION
To establish that facial dysmorphology can inform incisively on the developmental pathobiology of schizophrenia, it is necessary to show, in addition to its embryological relationship to brain structure, that variation in facial morphology reflects variation in adult brain function. Here we describe the results of such a study using cognitive measures.
Though we note that larger centroid size of the anterior face in young adults is associated with greater cognitive performance, this finding is unlikely to indicate simply that a larger overlying face reflects a larger brain and that a larger brain is associated with greater cognitive performance; rather, it is likely to reflect integration of the multiplicity of processes that determine the size of the cerebral-craniofacial complex and its functional capacity over the lifetime trajectory from early fetal life through to adulthood.
25,26 Only the early phase of this trajectory overlaps with the critical period during which cerebral-craniofacial shape and asymmetry are established.
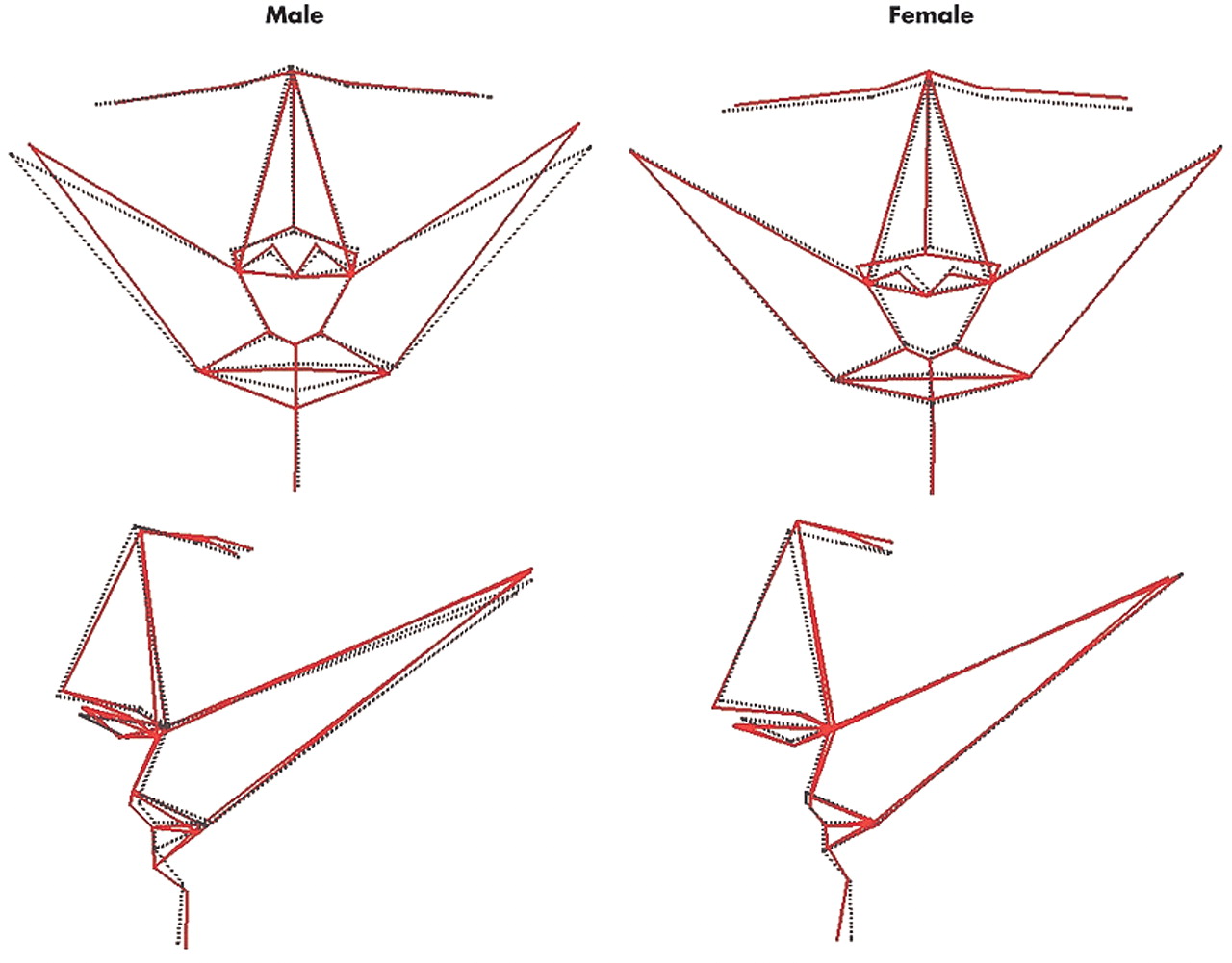
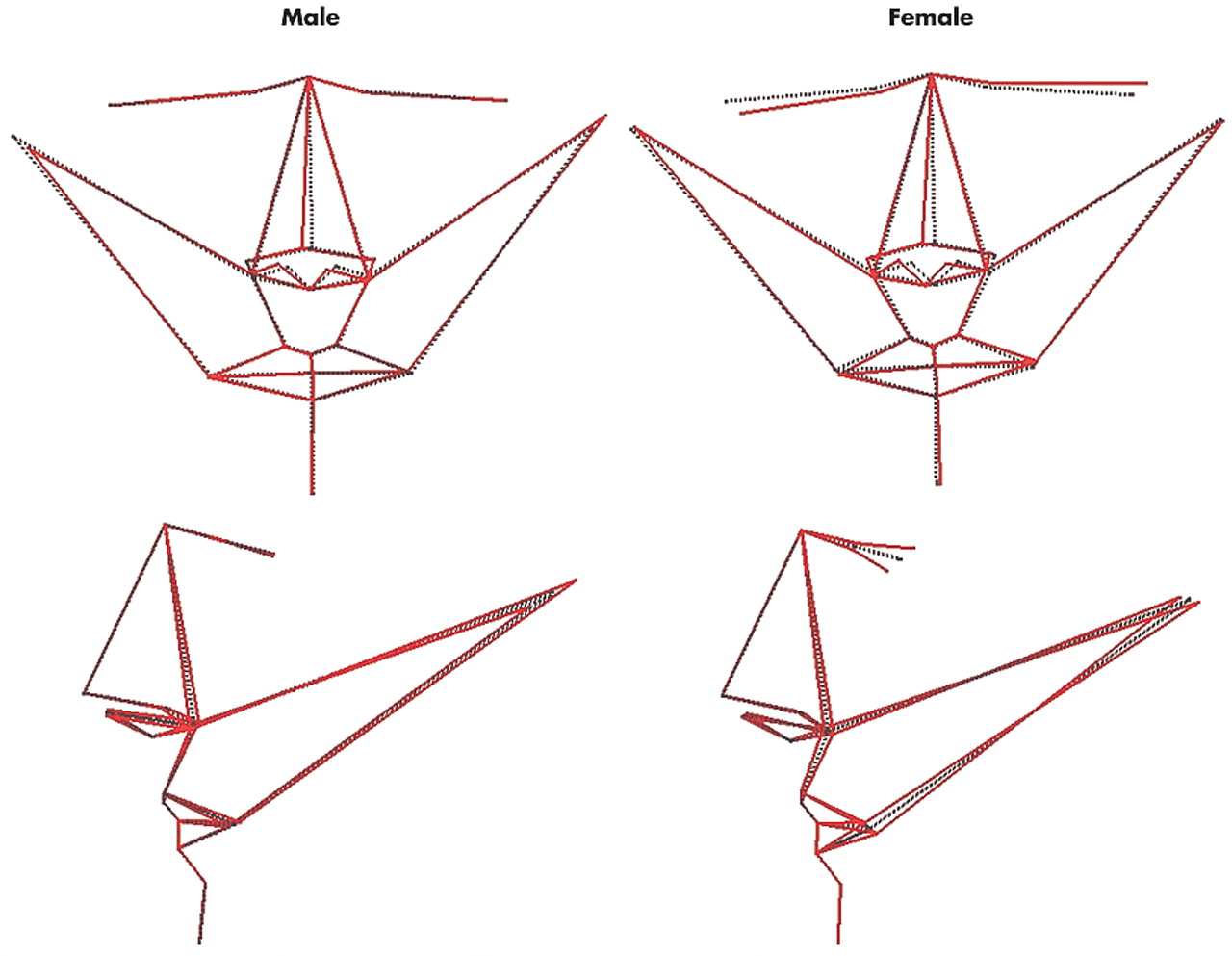
More fundamentally, we found that variation in anterior facial shape and asymmetry, independent of size, is associated with variation in cognitive performance in a sexually dimorphic manner. The magnitude of facial shape variation associated with cognitive performance is unlikely to be observed qualitatively in any individual by another individual. However, using three-dimensional laser surface scanning and geometric morphometrics, double dissociations were consistently found: overall shape and asymmetry of the anterior face covaried with spatial attention, visuomotor tracking, and processing speed in men but not women, and with verbal fluency in women but not men. Like visceral anatomy, the craniofacies and CNS develop along a primarily female trajectory unless there is male gonadal hormone secretion after gestational week 10 to initiate sexual dimorphism;
27 hence the sexes differ in cerebral morphology,
28,29 facial shape
16,25 and cognitive abilities.
28,30 Thus, it is striking that these morphological dissociations involve for each sex the cognitive domain in which that sex shows, at least in large samples, preferential overall performance: in general, men perform better than women on visuospatial tasks, while women perform better than men on verbal tasks.
28,30Furthermore, this sexual dimorphism in relationships between facial indices and cognitive measures is strikingly similar to that reported previously for relationships between brain structural indices and IQ in normal individuals.
39 In women, verbal IQ was found to correlate with the majority of brain structures examined (e.g., cranium, left and right cerebrum, cerebellum, left and right temporal lobes, left and right hippocampus), while correlations with performance IQ were limited; conversely, in men the majority of correlations were with performance rather than verbal IQ.
The present findings, relating particularly to shape and asymmetry of the frontonasal prominences, eyes, and mouth, involve primarily anterior facial structures which emerge together with anterior brain structures about the midline in a coordinated sequence. This process of anterior facial-cerebral development begins between gestational weeks 6 through 12 and is then established through the next several weeks of fetal life to attain the fundamental arrangement of facial features characteristic of an adult. Thereafter, ultimate attainment of adult form derives more from increasing size than from alteration in shape. Facial shape and size stabilize after adolescence and changes in later life are largely due to soft tissue movements. Since the subjects were adults with a mean age in the 30s and because landmarks are mostly determined by bone and cartilage, the present analyses should be insensitive to postadolescent facial growth. Furthermore, the absence of subjects over age 60 precludes effects related to any involutional or other changes over senescence.
More specifically, over this period of fetal life the anterior brain, neuroepithelium, neural crest, and facial ectoderm constitute a unitary, sexually dimorphic three-dimensional developmental process.
1–3,25,27 In contrast, development of the posterior face and brain are related less intimately and appear regulated by different mechanisms.
2,4 For each sex, there was a distinct topography of facial shape that varied with increasing performance in its associated cognitive domain: reduced head width, reduced width of the eyes, and thicker lower lips in men; higher and narrower upper face, wider and longer nose with reduced width and height of the mouth, and more prominent chin in women. That these distinct topographies of variation in anterior facial shape between the sexes have correlates in anterior brain function would be in accordance with the above developmental process and complementary to sexual dimorphism in anterior cerebral structure.
28,29It is known that the human face as well as the brain is intrinsically asymmetric, with extent of facial asymmetry being an important determinant of perceived attractiveness by others.
32 Though asymmetries are not identical mechanistically across all body regions, they share some regulatory processes,
33–35 thus facial and cerebral asymmetries are also established over early fetal life.
3,36,37 Critically, for each sex there was a distinct topography of anterior facial asymmetry that varied with increasing performance in its associated cognitive domain: nose set to the right of the midline, and head width increased on the left and decreased on the right in men; nose set to the right, and left eye and corner of mouth set high, back and to the left and the converse for the right eye and corner of mouth in women. These distinct topographies of variation in facial asymmetry between the sexes have correlates in brain function that are complementary to sexual dimorphism in cerebral asymmetry.
29,35,38,39CONCLUSION
The results of this study should be considered in relation to its strengths and limitations. Laser surface scanning and geometric morphometrics allow facial shape to be quantified and analyzed, both statistically and visually, in the three-dimensionality of the underlying developmental biology; in particular, these techniques allow for the independent resolution, analysis, and visualization of asymmetry, and its relationship to other psychological and biological variables. However, future studies should utilize a broader range of cognitive assessments to examine in greater detail the specificity of the functional correlates reported, and could apply these techniques to brain structure as well as function. Nevertheless, that the present findings took the form of double dissociations attests some robustness to the relationships reported.
Biological variation in events over early fetal life may be an important determinant of sexually dimorphic covariance of facial shape and asymmetry with aspects of cognition. After this critical period of development, many additional biological and psychosocial factors are likely to operate across the sexes in sculpting cognitive performance to the level attained in adulthood. However, among these, covariance of cognitive performance with facial shape remains evident long after the early events which established that shape. In preliminary studies using indirect reconstruction of facial configurations from linear anthropometric measurements, we have reported recently that subtle facial dysmorphology noted in schizophrenia
8–10 includes sexually dimorphic disruption to facial asymmetries,
14 in elaboration of disruption to cerebral asymmetries.
40 However, the extent to which such aspects of facial dysmorphogenesis reflect brain dysfunction has been unknown. The present study indicates that variation in facial shape and asymmetry reflects variation in adult brain function. This attests the approach of direct three-dimensional laser surface scanning of facial dysmorphogenesis for informing on the developmental pathobiology of psychotic and other illnesses and their clinical correlates. On this basis we are currently conducting such studies in schizophrenia. Studies in first-episode psychosis may be particularly informative.