The molecular basis of FXTAS is thought to be the elevated FMR1 mRNA observed in peripheral blood leukocytes of premutation carriers.
9 The FMR1 mRNA levels are elevated two- to 10-fold, possibly because of impaired translation of the expanded CGG-repeat mRNA with a consequent increase in transcriptional activity.
10 The elevated FMR1 mRNA is thought to produce a toxic gain of function effect whereby proteins that are important for neuronal cell function bind excessively to the expanded CGG repeat and are sequestered.
11 In patients with FXTAS, inclusions subsequently form in the nucleus of the neurons and astrocytes, which results in neural cell loss, particularly of Purkinje cells of the cerebellum.
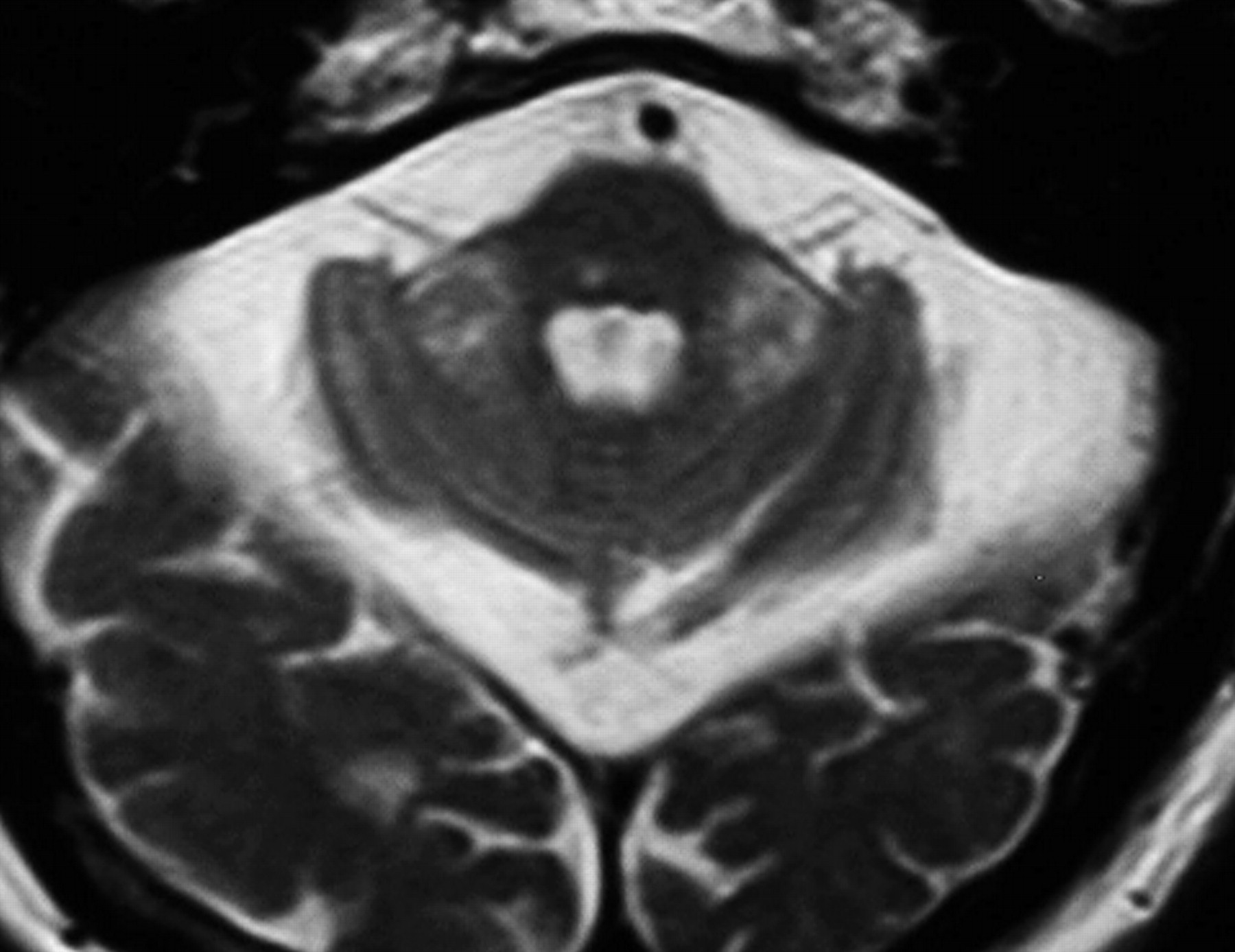
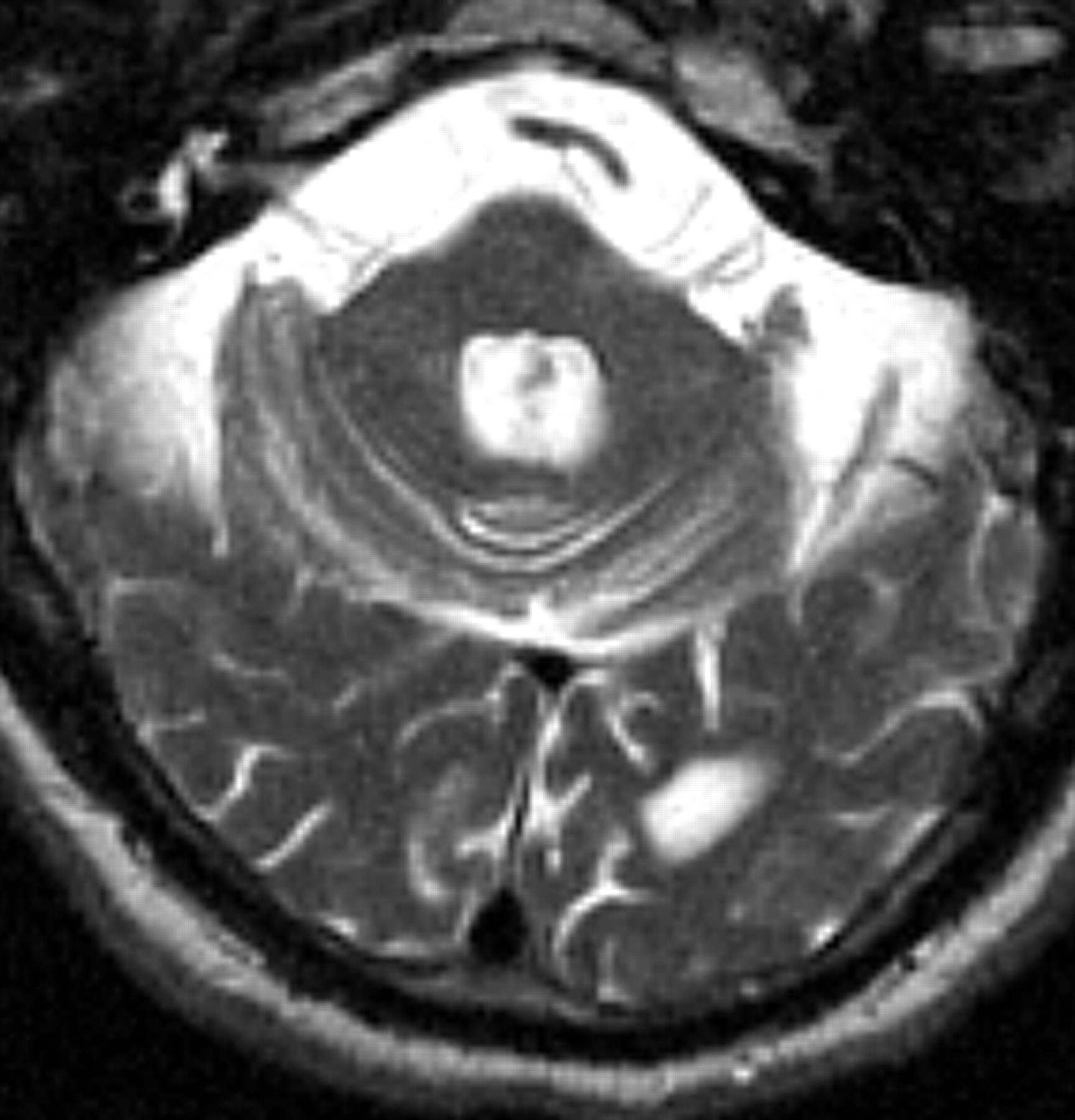
12 Cortical atrophy and white matter disease are seen on magnetic resonance imaging (MRI) in patients with FXTAS; in addition, over half of the patients show increased signal intensity on T2 weighted images within the middle cerebellar peduncles (MCP sign) and white matter of the cerebellum adjacent to the dentate nuclei (
Figure 1 ).
13CASE REPORT
In 2001, a 65-year-old white man with the fragile X premutation (98 CGG repeats) was reported for his neurological symptoms, including tremor and ataxia.
14 The onset of upper extremity tremor was estimated to have begun at age 54. Four years later, the tremor having worsened, he had to retire from his job as an electrician, a career at which he had been highly skilled and successful. Subsequently, the tremor intensified, impairing his activities of daily living (ADLs) and forcing him to rely on others regularly for assistance. He also experienced several falls from postural instability. He was treated with amantadine, which briefly improved his postural status but did not affect his tremors.
He had been married for 30 years and had three adult children with normal intellectual abilities and two grandchildren with fragile X syndrome, through whom his premutation was identified. He also had a brother with mental retardation and fragile X syndrome. In addition, there was a history of likely dementia in his father and Parkinson’s disease in his grandfather. His birth and early development were unremarkable: he functioned well in school, was a high school graduate with 2 years of college education, and then received electrician training, during which he was characterized as a “star pupil.” He had no history of alcoholism. Medical history included hypertension and hypercholesterolemia. He was also receiving lisinopril, carbidopa/ l -dopa, and lovastatin.
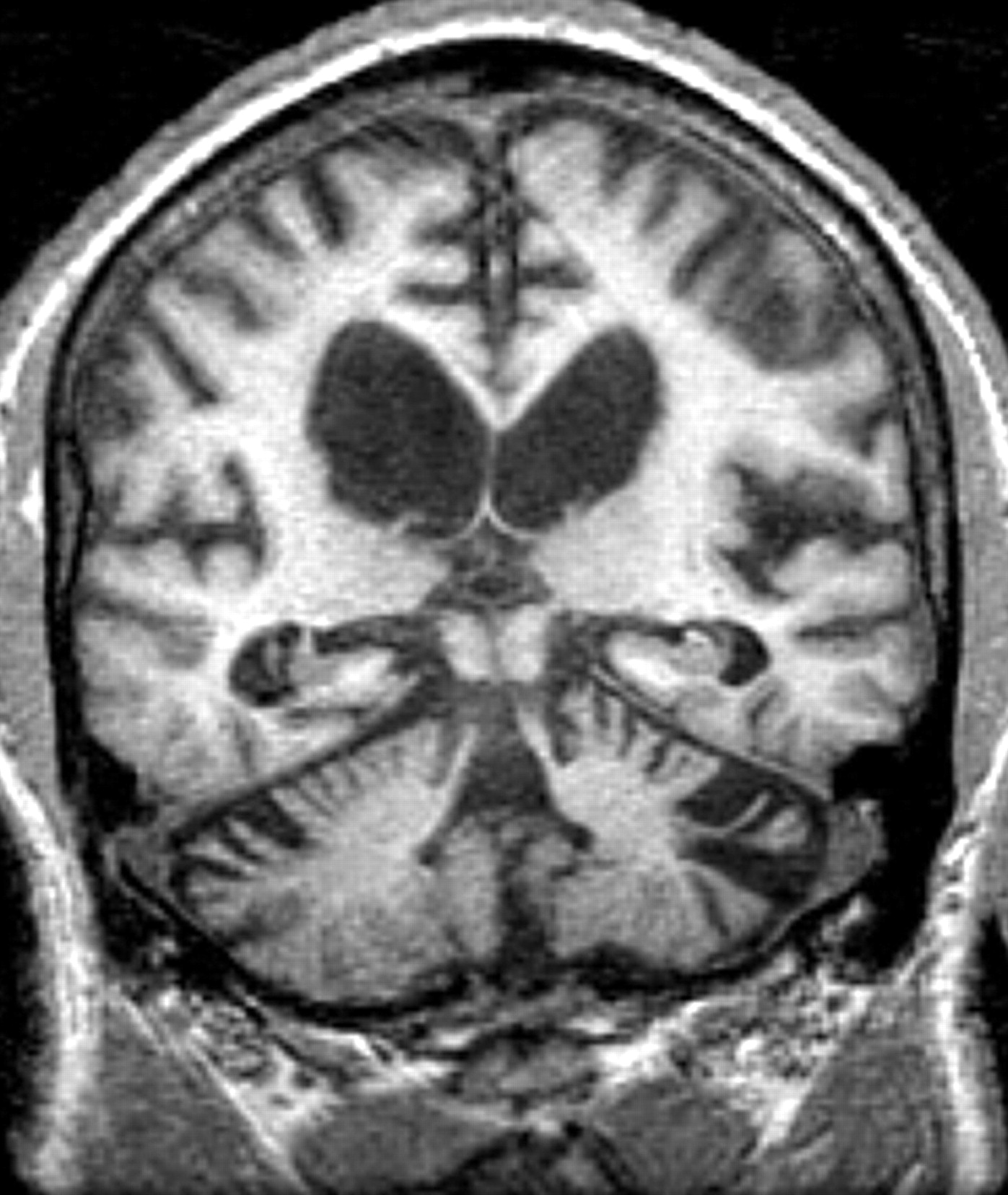
His psychiatric symptoms included slowness in initiation and completion of simple tasks, excessive sleep with daytime naps, fatigue, low levels of energy, poor concentration, and decreased psychomotor movements. His spouse reported that he had an irritably depressed mood, but the patient denied feeling depressed or sad and there were no clear reports of anhedonia. He had no history of psychosis or suicidal behavior. He had been diagnosed with cognitive symptoms, with notable poor memory for recent events and the names of familiar people, which became apparent several years after the onset of his tremors. An MRI in 2000 showed dilated lateral ventricles and cortical atrophy (
Figure 2 ). He had been started on donepezil 5 mg qd in 2001 without dramatic effect. This led to his referral for psychiatric evaluation in 2002.
Genetic testing revealed an expansion of 98 CGG repeats, an elevated FMR1 mRNA level (3.4 [SD=0.48] times above normal), and that FMR1 protein was reduced to 69%. Formal neuropsychological testing accomplished in 2000 revealed a Verbal IQ of 93, a Performance IQ of 73, and a Full Scale IQ of 83. Verbal comprehension was 100, perceptual organization was 78, working memory was 80, and processing speed was 71. His executive cognitive function was severely impaired, with a score of 3 on the Behavior Dyscontrol Scale (BDS),
16 and a score of 6 (normal score being 39 [SD=9.8]) on the Controlled Oral Word Association Test (COWAT).
16 Verbal fluency and processing speed also were impaired substantially, with a score of 28 on the Symbol Digit Modalities Test (SDMT-1)
17 and a score of 8 on the Animal Naming subtest of the Boston Diagnostic Aphasia Examination (BDAE).
18 During the testing, he demonstrated notable disinhibition and distractibility, inappropriate joking, poor frustration tolerance, and perseverative thinking. A more detailed neurocognitive assessment was reported in Grigsby et al.
19Psychiatric examination revealed notable psychomotor slowing, blunted affect with occasional smiling, and coherent thought process, albeit with some perseveration. There was notably long latency of verbal responses. Similarities and proverbs were interpreted concretely and in a somewhat idiosyncratic fashion. His knowledge of historical events was fair, but he exhibited some disinhibition (e.g., making disrespectful comments about various historical figures in an inappropriately jovial way) and lacked detailed understanding. There were no signs of suicidal or homicidal ideation and no evidence of psychosis. His tremors, discussed above, were evident throughout the psychiatric examination. Because of these tremors, writing and drawing tasks could not be accomplished on formal cognitive testing. His Mini-Mental State Examination (MMSE) score was 24, with decrements in short-term memory (only one of the three elements was recalled correctly) and time orientation (four of the give time elements were correct). Psychiatric diagnosis was dementia secondary to FXTAS.
Donepezil dosage was increased to 10 mg qd. Upon follow-up 4 weeks later, his spouse reported greater cognitive accuracy and improved memory. A repeated MMSE was 26, with improved short-term recall of two of three elements. He had less latency of verbal response. A Hamilton Depression Rating Scale (HAM-D) revealed a score of 3. His decreased psychomotor movements and spousal reports of recent irritability resulted in the administration of venlafaxine 37.5 mg XR qd. Within one month of combined therapy, he was less irritable and more attentive in conversation, had improved sleep and energy, and was showing better memory function in ADLs. He continued to deny depressed mood. Reexamination revealed increasingly spontaneous speech, less blunted affect, and an improved MMSE to 27, with short-term recall still at two of three elements. He continued on venlafaxine and donepezil. In subsequent months, he experienced brief episodes consistent with disturbed reality testing. On one occasion, he awoke at night and asked, “Where is my gun?”; on another occasion he expressed the belief that he “had to go to work.” Risperidone 0.5 mg po qhs PRN was prescribed thereafter but was taken infrequently.
Within the next 6 months, his neurologist discontinued carbidopa/l-dopa and prescribed amantadine. One year after his initial psychiatric evaluation, his MMSE was stable at 27 with two of three elements on short-term recall and his mood symptoms remained improved. This emotional and cognitive stability was noted despite significant increase in his tremor in the interceding months.
A complete neurological examination in 2003 revealed worsened tremors, ataxia, and parkinsonism. Cranial nerve examination revealed hypomimia (reduced facial expression), hypophonia (monotonous speech with some nasal quality), saccadic pursuit eye movements, and bilateral hearing impairment. Motor examination revealed a 4–6 Hz high amplitude postural tremor, which affected his upper extremities bilaterally; an intermittent resting tremor of the right thumb and index finger; and moderate to severe rigidity with some cogwheeling, which affected both upper extremities but was more pronounced on the left. He had bradyteleokinesia on finger-to-nose testing bilaterally. As a consequence, his handwriting was large, tremulous, and illegible. In addition, he was dysdiadochokinetic on fast alternating movements of hands and fingers, ataxic on heel-to-shin maneuvers, and he demonstrated a broad-based gait with mild propulsion and moderate hesitation. His deep tendon reflexes were hyporeflexic in all extremities, and his plantar responses were flexor bilaterally. On sensory examination, he had significant vibratory loss in the great toes bilaterally. Interestingly, however, his position sense was preserved in his lower extremities.
Regarding parkinsonism, he was tested based on the motor component of the Unified Parkinson’s Disease Rating Scale (UPDRS)
20 at each clinic visit. The motor component of UPDRS comprises 14 items dealing with the subject’s speech, facial expression, tremor, rigidity, postural stability, and body bradykinesia. It provides a numeric score for an objective measurement of the subject’s motor functions, with zero being completely normal and 56 the worst possible. Our subject scored 35 at his most recent visit in 2004, a 12 point decrement within 1 year, signaling significant deterioration of his overall motor capacity.
According to his neurologist, who conducted a follow-up visit in 2004, his ataxia had deteriorated to the point that he needed a wheelchair or walker much of the time. His tremors had increased in severity, rendering him unable to complete the Fingertapping Test and the Purdue Pegboard Test upon examination. He had been treated with primidone for tremors, which initially had been helpful but by this follow-up visit was less effective.
DISCUSSION
This is the first case report in the psychiatric literature of FXTAS, which appears to be a relatively common cause of tremor and ataxia among older men in the general population (approximately 1 per 3,000).
4 A recent prevalence study of families with FXS in California demonstrated that 17% of men with the premutation in their 50s, 38% in their 60s, 47% in their 70s, and 75% in their 80s develop FXTAS.
4 This case represents a constellation of neurological, psychiatric, and MRI findings that are characteristic of the recently described FXTAS in grandfathers of subjects with fragile X syndrome.
Anecdotal case reports of patients with FXTAS detail psychiatric symptoms including anxiety, mood lability, irritability, and reclusive social behavior. These symptoms may be secondary to the cognitive impairments or may be primary to the pathological process of FXTAS. The region of the brain with the highest percentage of inclusions in neurons and astrocytes is the hippocampus.
12 Psychiatric symptoms may be primary and related to limbic dysfunction.
21 Executive function deficits, often associated with impulsive behavior and inappropriate verbalizations, are also presenting signs, along with the tremor and ataxia.
7,
8 We have seen dementia in approximately 20% of patients with FXTAS, but longitudinal studies that may increase the percentage over time have not been performed.
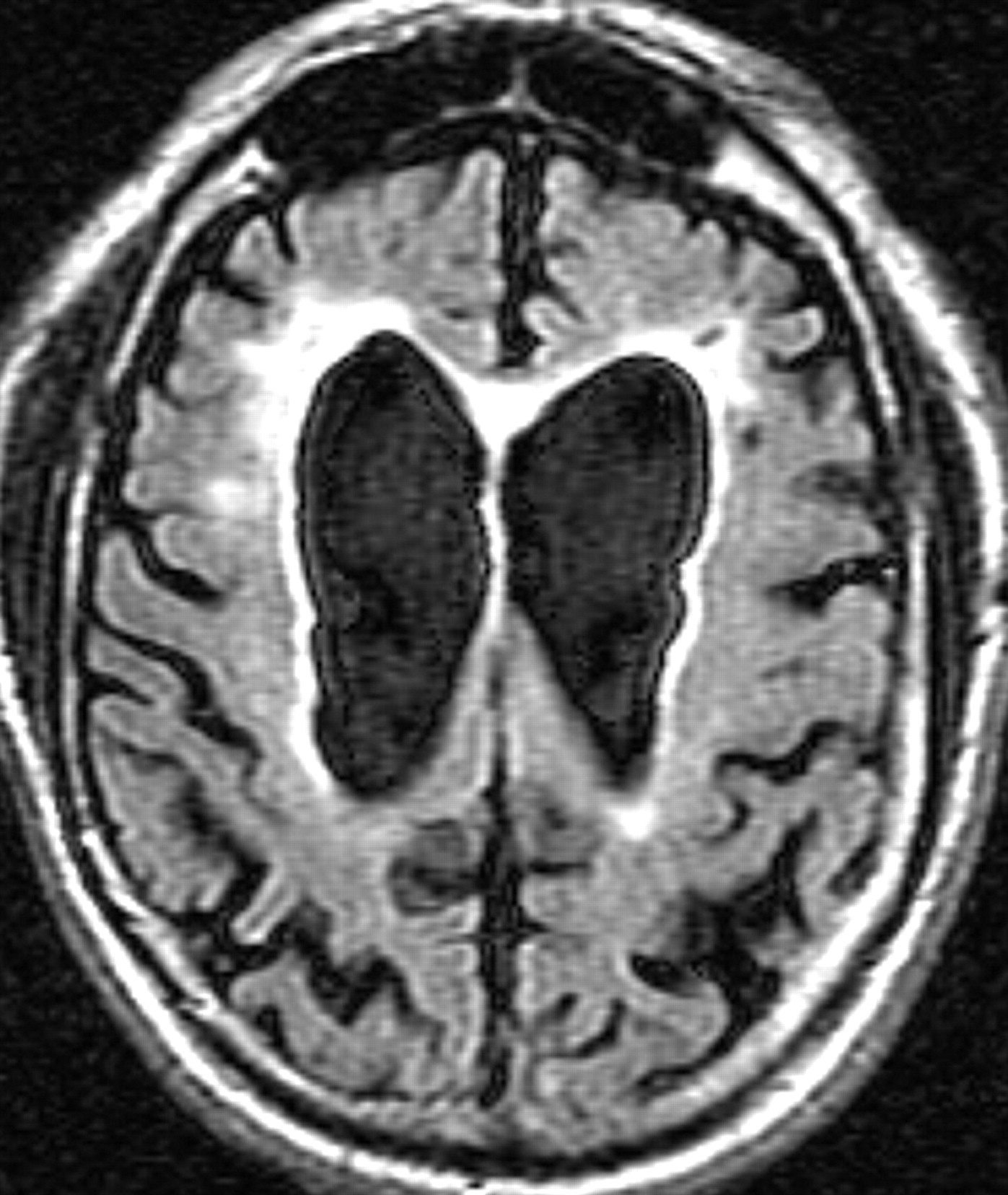
8The MRI usually reveals cortical atrophy, ventricular enlargement, and periventricular white matter increased signal intensity on T2 weighted images, which is presumably secondary to demyelination and gliosis associated with neuronal cell death. In addition, white matter disease is present in the middle cerebellar peduncles (MCP) in over 50% of patients, although this was not seen in the current case (
Figure 2 ).
The patient presented a pattern of psychiatric symptoms consistent with both subcortical and cortical (primarily frontal lobe) dementia. Consistent with subcortical pathology (as is often seen with Parkinson’s dementia and vascular dementia) were his cognitive slowness, poor retrieval memory, and associated mood symptoms that were subsyndromal for a major depressive episode, including variably depressed mood, energy disturbance, decreased psychomotor movements, and sleep disturbance.
22 Symptoms consistent with a deteriorating frontal lobe process were his behavioral disinhibition, poor social judgment, task impersistence, and poor ability to initiate activity, despite a relatively preserved MMSE score. His symptoms improved incrementally with treatment with an antidepressant, making speculative the specific syndromal attribution of depression “versus” dementia and the precise relative contributions of the cholinesterase inhibitor and antidepressant to his clinical condition.
While the neuropsychiatric phenomenology of this syndrome awaits full descriptive study, it is a reasonable assumption that the natural history of this degenerative condition would include linear decline in cognitive function, as is seen with dementia of the Alzheimer’s type (DAT). This stands in contrast to some other dementia syndromes, such as vascular dementia, where episodes of cognitive decline may be interspersed with periods of relative cognitive preservation, so that a patient’s decline is experienced as more “stepwise.”
22 While it is as yet unclear if the rate of cognitive decline in this syndrome would be a loss of 2 to 4 MMSE points/year (as in untreated DAT), this rate may have utility as a clinical benchmark of expected decline pending the completion of the necessary longitudinal studies to elucidate the natural history of the untreated condition.
22This patient was treated for 3 years with donepezil, an FDA-approved cholinesterase inhibitor to treat dementia of the Alzheimer’s type.
23 Since their introduction, cholinesterase inhibitors have been used “off-label” for other dementia syndromes (e.g., vascular dementia, Parkinson’s disease with dementia, and Lewy body dementia).
24 –
31 The patient tolerated donepezil without apparent side effects, showed modest cognitive improvement, and then maintained a relatively stable MMSE during 2 years of psychiatric treatment, when he otherwise may have been expected to lose cognitive function. This point remains, of course, somewhat speculative and needs to be validated by properly designed prospective studies.
Anxiety is a symptom that can be present often in premutation carriers even before the onset of FXTAS; typically, carriers with anxiety do well with a selective serotinin reuptake inhibitor (SSRI).
32,
33 The norepinephrine enhancement of venlafaxine may be beneficial for the attentional problems related to the executive function deficits in FXTAS. In addition, the patient experienced several mood symptoms (e.g., excess sleep, low level of energy, irritability, and lability) in addition to symptoms better understood as fundamentally “cognitive.” It is common psychiatric practice to use adjunctive antidepressant treatment with pharmacotherapy of dementia for concurrent mood and cognitive symptoms.
34 –
37 While this patient never met clinical criteria for major depressive disorder during the 2 years of psychiatric treatment, he did appear to experience mood symptoms that affected his already compromised cognitive function. He tolerated venlafaxine without problematic side effects and experienced improved cognitive and behavioral functioning.
Further studies of helpful medications in FXTAS are warranted since this disorder is common. We also urge DNA testing for the FMR1 premutation in patients who present with dementia and psychiatric symptoms, in addition to tremor and/or ataxia.