A ggressive behavior is a frequent sequela of traumatic brain injury. In 1981, McKinley et al.
1 reported a 70% incidence of posttraumatic irritability of which 20% was defined as violent behavior that could persist for at least 5 years after injury.
2 Aggression following head trauma is often poorly directed, unrelated to any specific trigger, and can occur with minimal or no provocation.
3 –
5 It is often attributed to a loss of behavioral self-control that reflects damage to or dysfunction of orbital and ventro-medial structures
6 and poses a serious challenge to rehabilitation and community reintegration, sometimes leading to long-term institutional placement.
4 Aggression after head trauma also has been associated with domestic violence
7 and is a factor implicated in relationship failure after head trauma.
8 Studies on criminals with a history of violence
9 and on juveniles and adults who display antisocial and violent behavior
10 –
12 have reported an association between aggression and neuropsychological dysfunction. Stanford et al.
13 matched 12 college students who reported a history of impulsive aggression with 12 non-aggressive student comparison subjects and found differences in cognitive performance on two tests that involve frontal control functions (Trails B and Wisconsin Card Sorting test). Aggressive students also exhibited deficits on tests of verbal ability that were interpreted as weakened strategic thinking, which results in impulsive aggression. A meta-analytical review conducted by Morgan and Lilienfeld
11 supported a relationship between antisocial behavior and neuropsychological measures of executive function, as did reviews conducted by Brower and Price
14 and Paschall and Fishbein.
15Little information is available on neuropsychological correlates of aggression after head trauma. Greve et al.
16 compared 45 residents at a center for people suffering long-term disability following traumatic brain injury. Twenty-six residents had a history of aggression and 19 were non-aggressive. Those with posttraumatic aggression had a premorbid history of aggression, impulsivity, irritability, and antisocial behavior, compared to nonaggressive comparison subjects. There were no cognitive differences between the two groups. Greve et al.
17 attributed this to the fact that a) patients used in their study were more cognitively impaired and more variable in their performance than non-brain-injured samples previously reported; b) deficits previously recorded on measures of higher order cognition (e.g., Stanford et al.)
13 were not easily identified in the context of serious head trauma; c) that orbitofrontal and ventromedial damage implicated in disorders of self-regulation are not easily identified by many traditional neuropsychological tests.
18,
19Our aim in this study was to compare the neuropsychological and neurobehavioral profiles of individuals who display posttraumatic aggression with a nonaggressive brain-injured comparison group. Because of the vulnerability of frontal structures to head trauma, we hypothesized that patients who display characteristics of aggression would exhibit significantly more verbal and executive deficits, and more neurobehavioral problems of an impulsive or disinhibited character, than patients with similar injury severity but without aggressive behavior. Further, we expected that such problems would lead to greater adjustment difficulties in terms of employment and relationships.
METHOD
Full ethical approval was obtained from the Department of Psychology, University of Wales, Swansea, United Kingdom, and the Local Research Ethics Committee of Swansea NHS Trust. In all cases, informed consent was obtained after the study procedures had been fully explained to the participants.
Participants
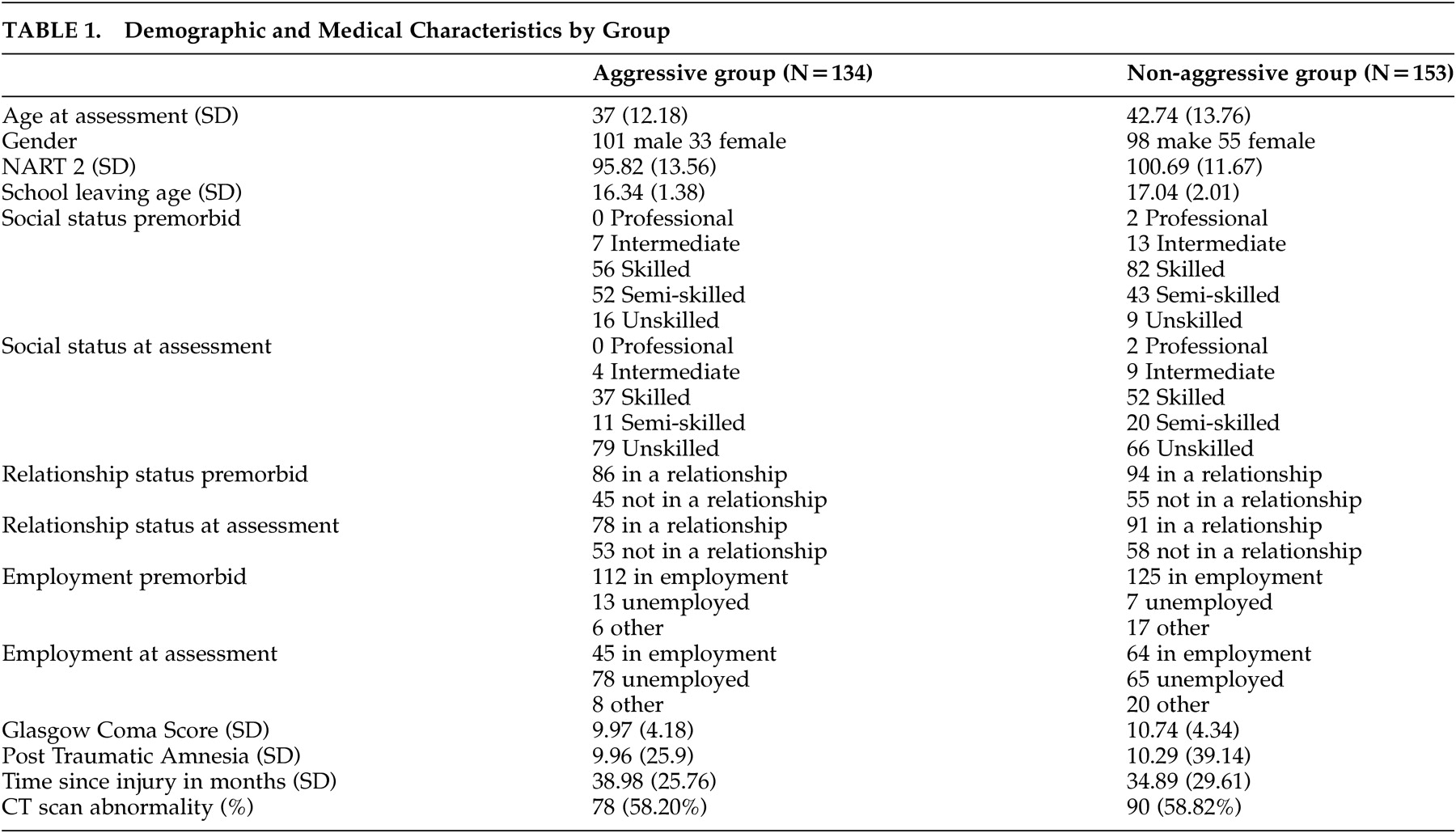
Over a 7-year period (March 1997 to June 2004), 287 severely head-injured patients referred for neuropsychological examination and rehabilitation advice were selected from 361 consecutive referrals. Exclusion criteria included a) a previous history of head injury, neurological or psychiatric disorder; b) alcohol or drug abuse; c) neurological or neuropsychological disability, such as speech, motor or perceptual deficit, likely to interfere with neuropsychological assessment; and d) a pre-accident history of aggressive behavior. Twenty-nine cases were excluded from the cohort based on those criteria. Patients were seen between 1 and 3 years post-injury. All were living independently in the community, but experienced persisting employment and relationship difficulties as a result of the cognitive and behavioral legacies of their head injuries. Demographic and injury details are presented in
Table 1 . Those cases with independent neuroradiological reports of abnormal computed tomography (CT) brain scans (78 in the aggressive group, 90 in the nonaggressive group) had mainly suffered frontal hemorrhagic or contusion-like injuries.
We assessed the incidence and nature of aggressive behavior during a semi-structured clinical interview. Reports from family members and patients were corroborated by case records (such as from social worker, general practitioner, police or employment records). We identified 134 cases with post-accident histories of verbally abusive and threatening behavior, or actual physical violence. The frequency and severity of this behavior varied from case to case, but had persisted for at least 2 years after injury and was out of character with the patient's history before the accident. Only a small number, 14 (10.68%), had been involved with the police because of violent behavior, but the majority of partners stressed that relationships were extremely fragile as a result of impulsive aggression or unpredictable and volatile changes in mood and temperament.
The groups did not differ significantly in severity of injury, indexed by Glasgow Coma Score
26 [t (109)=−0.95, p=0.34; η
2 =0.003] and duration of posttraumatic amnesia [
t (281)=−0.08, p=0.93; η
2 =0.05]; time between injury and neuropsychological assessment [t (285)= 1.25, p=0.21; η
2 =0.005), or CT scan evidence of brain injury (χ
2 =0.01, p=0.92, Cramer's V=0.006).
Design
In this prospective cohort study, a quasi-experimental approach, using between-groups analysis of z scores derived from the whole cohort, evaluated relative differences in cognitive function between brain-injured patients with and without aggression. Age-corrected z scores were derived from normative data on each measure to establish the cognitive profile of the aggressive group.
Measures
Injury severity was based on clinical records indicating Glasgow Coma Score <12 on admission to the hospital, plus posttraumatic amnesia >24 hours. Posttraumatic amnesia duration was ascertained according to the guidelines proposed by McMillan et al.
20 The social class of each participant was coded on the basis of their pre-injury occupation, using the Office of Population Censuses and Surveys Classification of Occupations.
Patients were administered a battery of neuropsychological tests, including the Wechsler Adult Intelligence Scale (WAIS III),
21 Wechsler Memory Scale (WMS III),
22 National Adult Reading Test–Revised (NART-R),
23 Hayling and Brixton Tests,
24 Trail Making Tests (TMT),
25 Speed and Capacity of Language-Processing Test (SCOLP),
26 and the Zoo Map test from the BADS battery.
27 To assess depression and anxiety, patients were given the Beck Anxiety Inventory
28 and Beck Depression Inventory.
29 Measures were classified a priori to represent theoretically determined cognitive domains as follows:
•
Language: vocabulary, similarities, comprehension
•
Visuospatial ability: Block Design, Matrix Reasoning
•
Mental speed: Digit Symbol, Symbol Search, Trails A, the Speed and Capacity of Language-Processing Test
•
Logical Memory 1, Logical Memory 2, Verbal Paired Associates 1, Verbal Paired Associates 2, Auditory Recognition Delayed
•
Working memory: Digit Span, Spatial Span, Letter-Number Sequencing, Arithmetic
•
Visual memory: Family Pictures 1, Family Pictures 2, Face Recognition 1, Face Recognition 2
•
Executive function: Hayling and Brixton, Zoo map, Trails B, Picture Arrangement
We used semi-structured interviews based on the Neurobehavioral Rating Scale
30 to elicit signs of neurobehavioral disability, and assessed the nature and impact of aggression using a behavioral checklist
4 that included both physical (e.g., pushing, shoving, hitting) and nonphysical (threatening speech, verbal insults, facial expressions) characteristics. We considered it more appropriate to collect data by interview instead of a standard paper-and-pencil questionnaire because of the sensitive nature and distressing effects of the behavior. Reliable quantification of frequency proved impossible because of the impulsive, unpredictable, and inconsistent pattern of aggression over time, but both patients and relatives declared that the behavior imposed major stresses on family relationships that were not present prior to injury.
Statistical Analysis
An a priori power analysis indicated greater than 95% power to detect differences of magnitude 0.25 between groups for a sample size of 210 (effect size (medium)=0.25, Critical
F (1, 208)=3.89, λ=13.12; GPower
31 ). All analyses were two-tailed, with α level set at 0.05. We controlled type I error with the Bonferroni correction for each family of tests. For both experimental and comparison groups, the percentage of scattered missing data for continuous independent variables ranged from 6.0% to 9.0%. Data were found to be missing completely at random, determined by Little's test
32 (experimental group: χ
2 (1529)=1619.91, p>0.05; comparison group: χ
2 (1711)=1797.59, p>0.05). Missing values for continuous independent variables were imputed using the expectation maximization method.
33Profile analysis, a specific style of multivariate analysis of variance (MANOVA), assessed differences in neuropsychological profiles between groups. To produce commensurability of the dependent variables, we calculated z scores, referencing all subjects in the study using the observed means and standard deviations of each test, from raw scores for all tests. This method allows for more variance in scores than z scores based on normative data in an impaired population. It also accounts for the interpretative difficulty of normative data being referenced to different normative samples for different tests. Composite z scores were formed for each of the cognitive domains described above by taking the mean of the z scores for all tests and for each subject represented in that composite. The profile analysis was then performed on these scores. The use of z scores imposed flatness on the data; therefore, only tests of levels and parallelism are reported in this analysis.
Using age-corrected z scores based on published normative data, we conducted additional analyses aiming to assess the profile of brain-injured individuals presenting with aggression in a manner similar to standard clinical practice. In this case, the z score represents the extent to which each subject deviates from the norms for his or her age. The mean of the patient's age-appropriate reference from the standardization sample was subtracted from the patient's test score and the result was divided by the standard deviation of the subject's age-appropriate reference group, giving a z score for each test score. Due to insufficient data presented in the manual, Hayling and Brixton approximate z scores were calculated based on the equivalence between z scores and percentile scores (Lezak, 1995). z scores were not estimated for SCOLP and Zoo map due to insufficient data provided in the respective manuals. A 90% confidence interval was chosen as a compromise level between the two levels recommended for the WAIS-III: 85% and 95%. All analyses were conducted using SPSS version 12.
34RESULTS
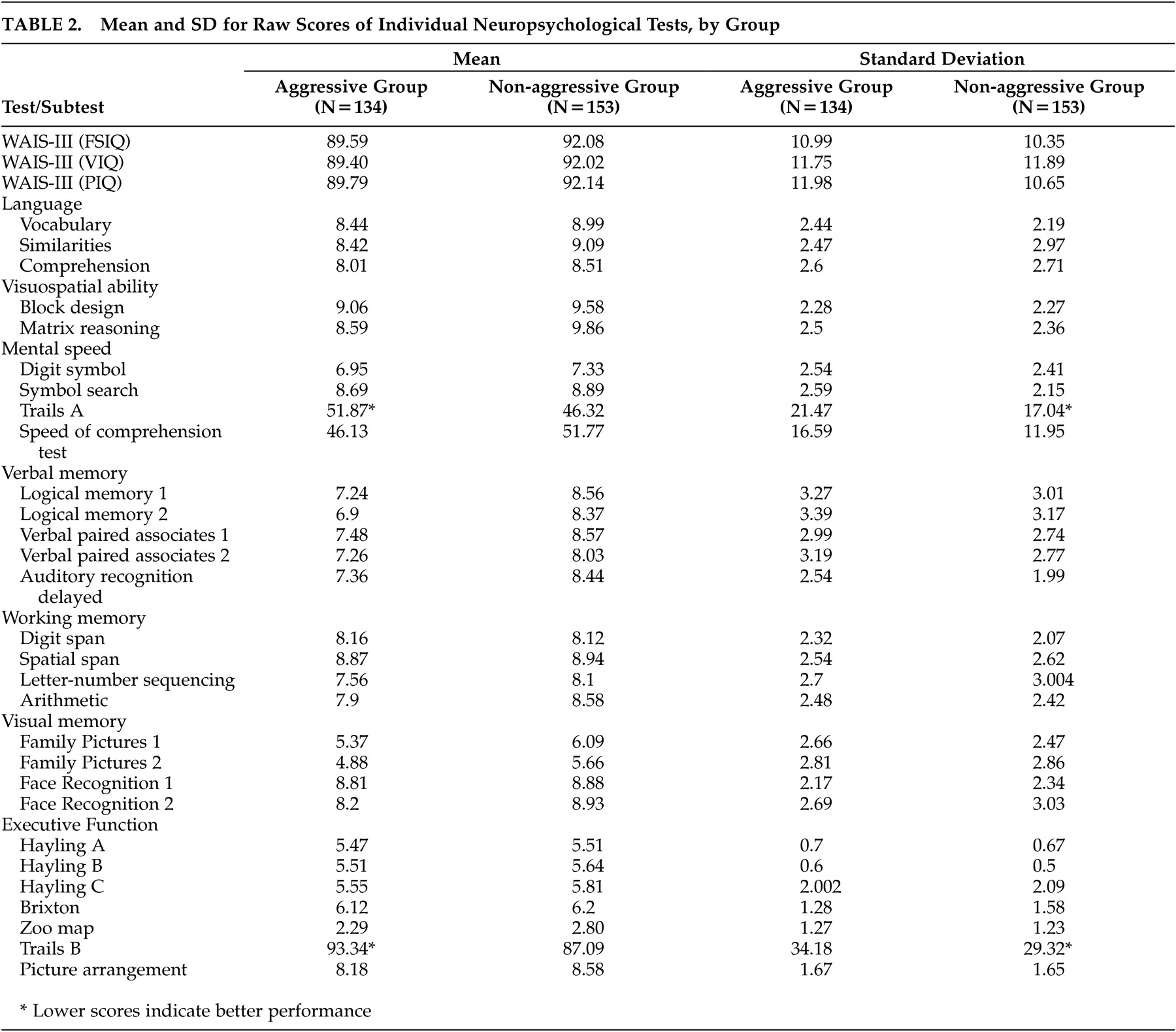
Table 2 provides raw score means and standard deviations for all neuropsychological measures. Aggressive patients had lower NART-R scores (an estimate of premorbid intelligence); [t (215)=–2.84, p=0.005; η
2 =0.03], left school earlier [t (262.75)=–3.43, p=0.001; η
2 =0.04], and were younger [t (284)=–3.71, p=0.001; η
2 =0.05] than the comparison group. Men were overrepresented in the aggression group (χ
2 =3.79, p=0.05, Cramer's V=0.12) and were of lower premorbid socioeconomic status (χ
2 =16.32, p=0.006, Cramer's V=0.24); they also maintained lower socioeconomic status after injury (χ
2 =13.09, p=0.02, Cramer's V=0.22). There was an overall difference in the premorbid employment status of aggressive and nonaggressive patients (χ
2 =9.65, p=0.04, Cramer's V=0.19). After injury this difference disappeared (χ
2 =8.93, p=0.06, Cramer's V=0.18). There was no significant difference in relationship status between the two groups before (χ
2 =5.24, p=0.63, Cramer's V=0.14) and after the injury (χ
2 = 4.41, p=0.73, Cramer's V=0.13).
A one-way between-groups multivariate analysis of variance (MANOVA) was performed to investigate group differences in Full Scale, Verbal and Performance IQ. There was no significant difference between groups on the combined dependent variables ( F [2, 284]=1.95, p=0.14; Wilk's Lambda=0.99, η p 2 =0.01).
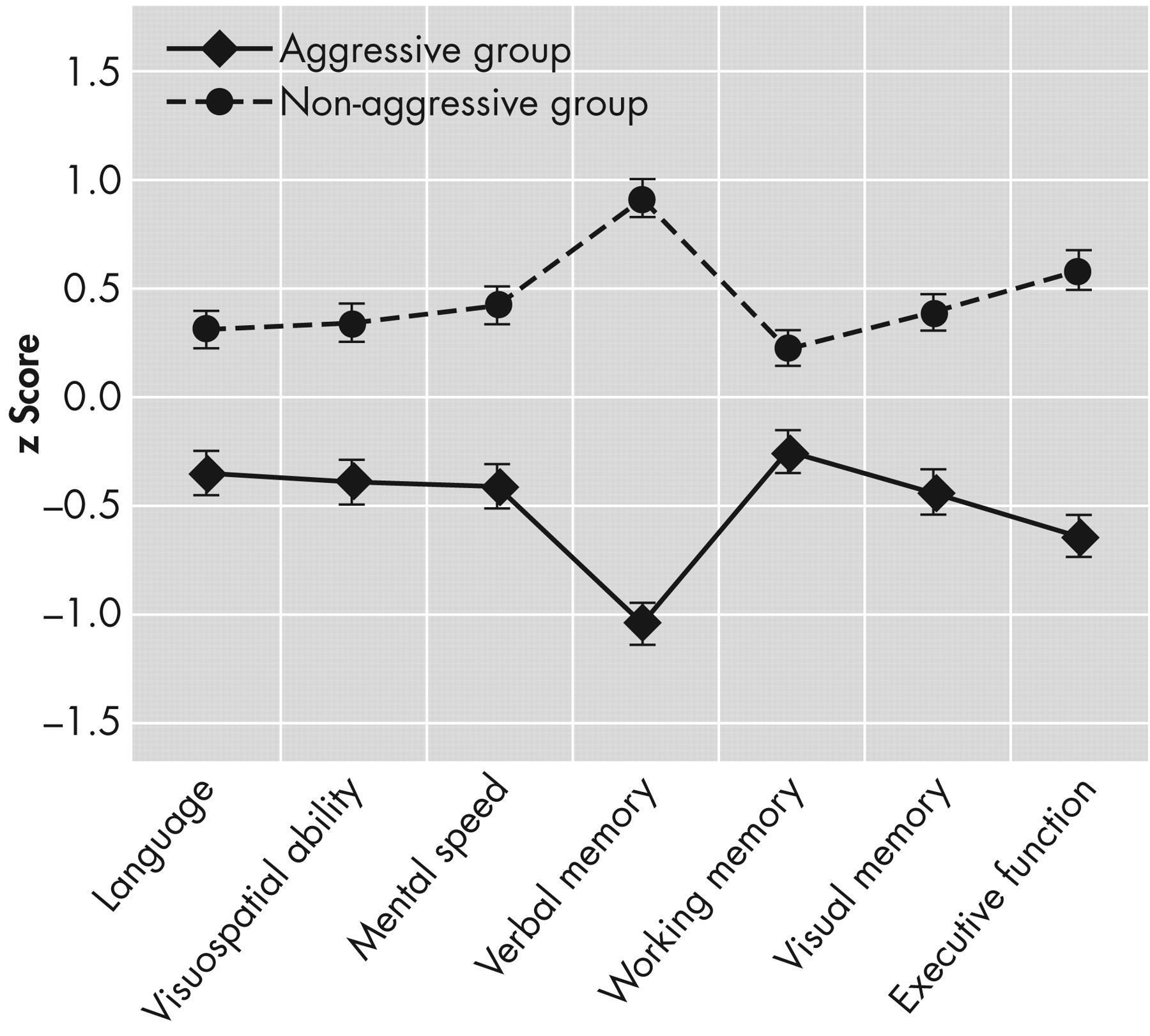
Group Differences in Composite Cognitive Domains
Profile analysis revealed significant differences between groups for the test of levels (F=9.25, df=1, 285, p<0.05, η
p 2 =0.03). Aggressive patients performed at a significantly lower level on cognitive domains than the comparison group. The test of parallelism, using Wilk's Lambda, was also significant (F=3.41, df=6, 280, p<0.05, η
p 2 =0.07), indicating that the pattern of scores can be considered significantly different for the two groups, after accounting for the overall effect of levels. Contrasts in the form of one-way ANOVAs following the profile analysis revealed that only the domains of verbal memory (F=13.99, df= 1, 285, p<0.007, η
p 2 =0.05) and visuospatial abilities (F=12.62, df= 1, 285, p<0.007, η
p 2 =0.04) significantly differed between the two groups.
Figure 1 depicts the profile of performance of each group across each cognitive domain.
Group Differences in Performance on Individual Cognitive Tests
MANOVAs conducted within the cognitive domains that were significantly different between groups showed that the following tests reached statistical significance—in
visuospatial abilities : Matrix Reasoning (F=19.46, df=1, 285, p<0.05, η
p 2 =0.06) and Block Design (F=3.78, df=1, 285, p<0.05, η
p 2 =0.01);
verbal memory : Logical Memory 1 (F=12.6, df=1, 285, p<0.01, η
p 2 =0.04), Logical Memory 2 (F=14.37, df= 1, 285, p<0.01, η
p 2 =0.05), Verbal Paired Associates 1 (F=10.26, df= 1, 285, p<0.01, η
p 2 =0.03), and Auditory Recognition Delayed (F=16.32, df= 1, 285, p<0.01, η
p 2 =0.05).
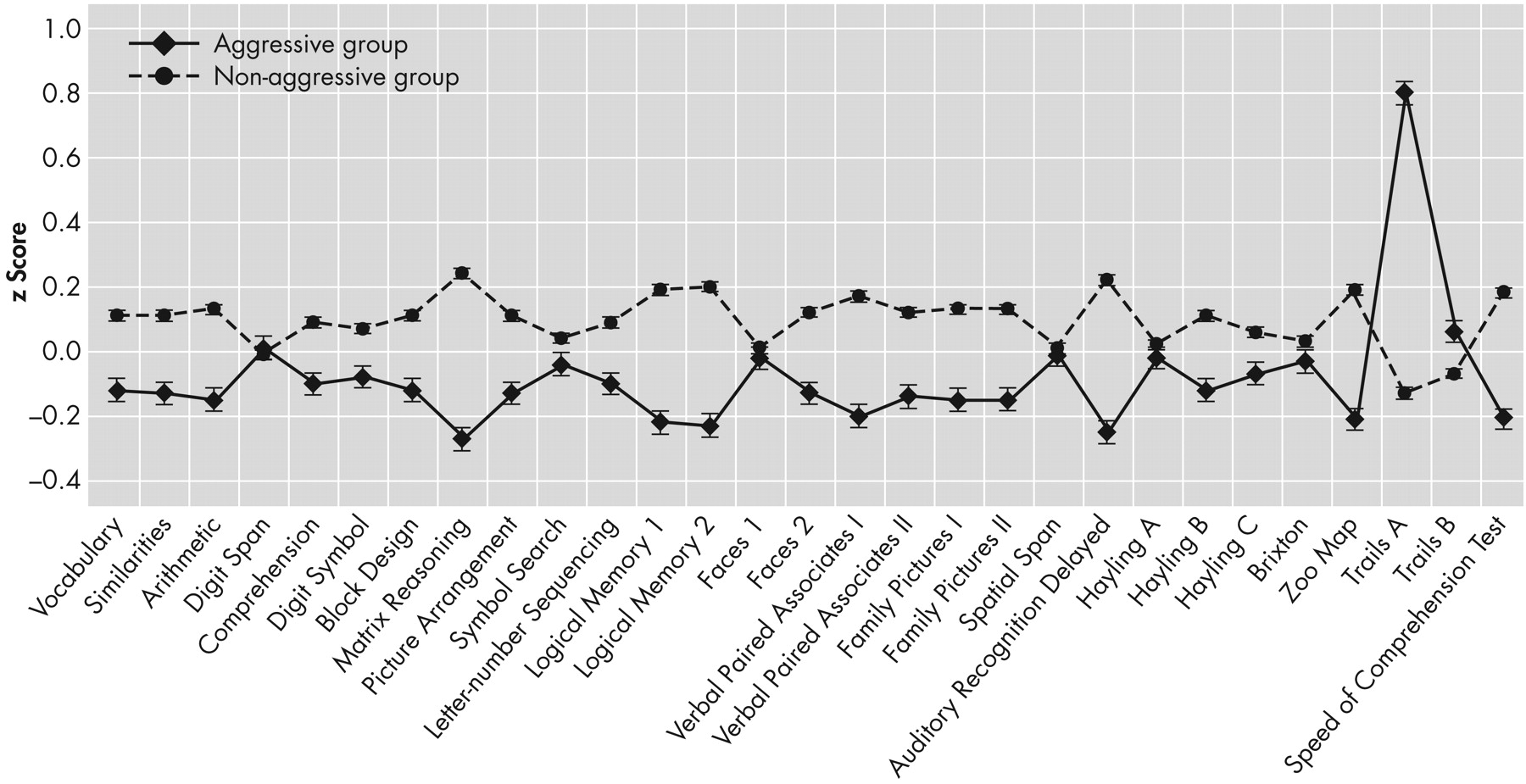
Figure 2 depicts each group's performance profile across each individual test.
Performance of the Aggressive Group Compared to Normative Data
The aggressive group performed within the normative range on most neuropsychological tests. Salient (≥1 standard deviation) deficits were observed in Trails A and B, Digit Span, Logical Memory 2, Family Pictures 1, and Family Pictures 2.
Figure 3 presents z scores for all measures.
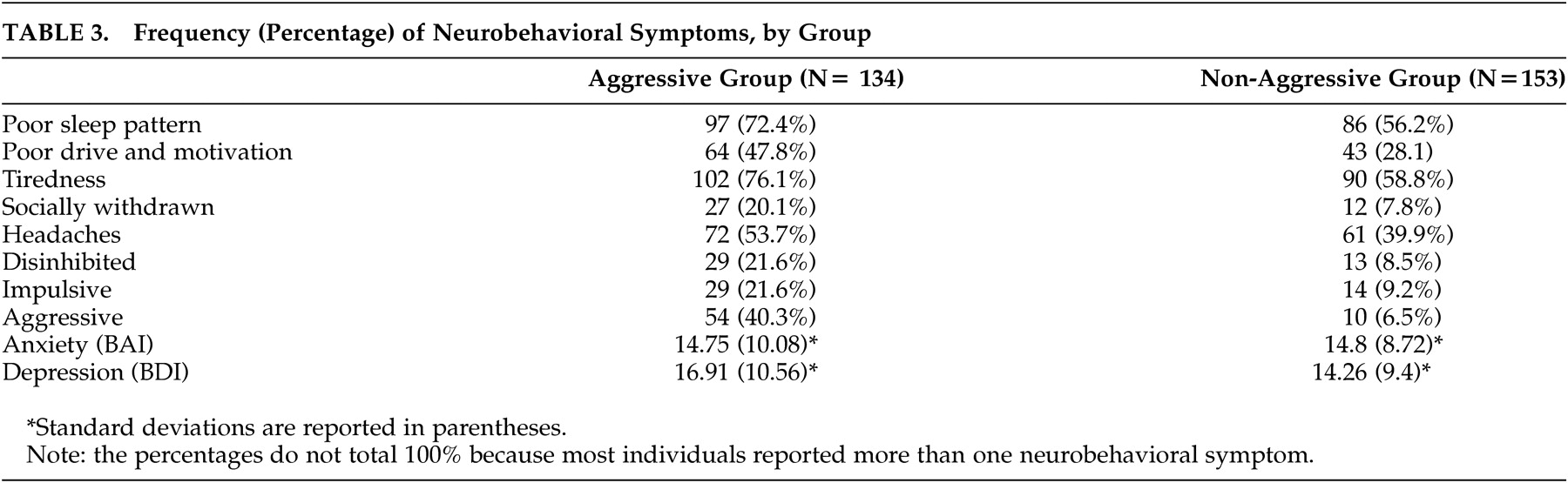
Neurobehavioral Correlates of Aggression
We found a significant between-groups difference in the incidence of these neurobehavioral symptoms: impulsive (χ
2 =7.91, p<0.007, Cramer's V=0.18), disinhibited (χ
2 =8.81, p<0.007, Cramer's V=0.18), socially withdrawn (χ
2 =8.15, p<0.007, Cramer's V=0.18), tiredness (χ
2 =9.07, p<0.007, Cramer's V=0.19), poor drive and motivation (χ
2 =10.97, p<0.007, Cramer's V=0.2), and poor sleep patterns (χ
2 =7.50, p<0.007, Cramer's V=0.17). There was no difference between groups on headaches (χ
2 =4.95, p=0.02, Cramer's V=0.02), levels of anxiety [t(157.22)=–0.04, p=0.97; η
2 = 3.5
−06 ] and depression [t (176)= 1.77, p=0.07; η
2 =0.01].
DISCUSSION
Our study aimed to establish the neuropsychological and neurobehavioral profiles of individuals who develop aggression following serious traumatic brain injury. Overall, the cognitive performance of this group was impaired relative to comparison subjects, albeit minimally, as indicated by the small effect sizes. An interesting finding was the relative specificity of deficits exhibited by the aggressive group in verbal memory and visuo-perceptual skills. A number of possible explanations can account for these results.
Our hypothesis that dominant hemisphere dysfunction is reflected by an impairment of verbal memory is partially supported by Yeudall's report
35 that violent individuals show relative dysfunction on neuropsychological tests that measure anterior left hemisphere functioning. Impaired visuospatial abilities suggest that the group's ability to analyze complex visual stimuli may be compromised. Using fMRI methods, Ruff et al.
36 found that the higher a person's visuospatial skills, the better their reasoning performance. Visuospatial reasoning activated anterior cingulate, medial, and dorso-lateral cortices, brain structures that are typically involved when several items in working memory need to be monitored and manipulated, such as when exploring strategies to control aggressive impulses.
37 The prefrontal cortex selectively mediates the ability to hold goals in mind while exploring and processing secondary goals,
38 an ability intrinsic to the process of planning and reasoning. The amygdala, which is particularly implicated in defensive aggression, also forms part of the complex circuitry mediating aggression. Brain injury can alter the balance between the potential for impulsive aggression, mediated by temporo-limbic structures such as the amygdala, and the control of this impulse via orbitofrontal mechanisms. It is for this reason that many individuals who exhibit aggression after brain injury are assumed to lack regulatory control over social behavior, implying an executive deficit. In our study, an indication of impaired executive-attention function in the aggressive group is provided by poor performance on Trails A, B and Digit Symbol tests, and the distinct neurobehavioral profile of the aggressive brain-injured group. Similar impairments have been identified in perpetrators of domestic violence.
39Verbal ability plays a critical modulatory role in aggression. Individuals with weak verbal skills are limited in their ability to use verbal mediation to resolve interpersonal conflicts, especially if they are impulsive.
9,
40 Though we found that aggressive individuals had lower language abilities compared to healthy subjects, the differences failed to reach statistical significance. A possible explanation is the conservative correction factor applied to control multiple comparison error. This practice, though it protects against type I error, increases the chances of a type II error.
As we hypothesized, the neurobehavioral profile of the individuals with aggression was characterized by significantly higher impulsivity, disinhibition, social withdrawal, and poor drive and motivation. This constellation of symptoms also reflects impaired executive function, despite the lack of impairment displayed by the group on recently developed specialized tests of executive function, such as the Hayling and Brixton tests. However, the ecological validity of these tests has recently been questioned in severely brain-injured patients.
19Our hypothesis that individuals with aggressive characteristics would have poor adjustment in terms of employment and relationship status was not supported. This counterintuitive finding is consistent with a recent study on personal relationships post-head injury,
8 which found that the unpredictability of aggression, not aggression per se, was the factor imposing the greatest burden on personal relationships and the best predictor of relationship breakdown after brain injury. However, it is surprising that aggression did not have a greater impact in an employment setting. Anecdotally, many individuals stated that they had secured jobs that required minimal social interaction where they could work at their own pace. Other individuals reported that they were able to contain their anger in work, only releasing it when they returned home (to the detriment of family life).
In line with other studies investigating aggression in clinical and normal populations,
9,
16 we found a relationship between low IQ, socioeconomic status, male sex, and aggression. It seems that these are predisposing factors in the development of aggression after brain injury. In early childhood, those with lower intellectual functioning are prone to develop aggressive behavior because of difficulty learning more complex, pro-social interpersonal skills. Aggressive behavior may, therefore, result in failure to develop intellectually because it can minimize opportunities for effective education.
Aggression is a complex social behavior and its cause is multifactorial. We cannot assume a simple association between brain dysfunction, or specific cognitive impairment, and aggression. More importantly, such an association does not imply causation. Specific lesions or diffuse injury may alter the threshold for aggression, but cannot be considered a sole direct cause. Modern neuropsychological theories acknowledge that complex behaviors are not the result of single localized brain areas but of complex interactions within the brain and between the brain and external environment. Our use of a quasi-experimental and clinical approach has highlighted different neuropsychological characteristics of individuals who show aggression after brain injury, but only provides hypotheses regarding the pattern of cognitive deficits associated with the development of aggression. A longitudinal study of healthy individuals who suffer brain injury and subsequently become aggressive is desirable for the purpose of early identification and treatment of individuals who are prone to violent acts after brain injury.