A lthough the pathophysiology of bipolar disorder is unknown, many investigators have proposed that it may relate to abnormal functioning of brain circuitry, particularly circuitry involving the basal ganglia. Such basal ganglia circuitry involvement is suggested by imaging studies of bipolar disorder.
1 –
6 Also, bipolar disorder patients have been reported to have a greater risk for developing drug-induced movement disorders, such as Tourette’s, than schizophrenia patients,
7 and the presence of catatonia, a motor disorder, is more likely to occur in the context of bipolar disorder than in schizophrenia or unipolar depression.
8,
9 Collectively, these observations implicate an abnormality of the dopamine system in the pathophysiology of bipolar disorder.
We considered that if disturbances in basal ganglia functioning are important in bipolar disorder, then it is possible that patients with the condition might show abnormalities in aspects of motor physiology that have been considered to relate to the functioning of these structures. Over the years we have developed quantitative instrumental approaches to measuring abnormalities in motor function that appear to be related to basal ganglia dysfunction.
10 –
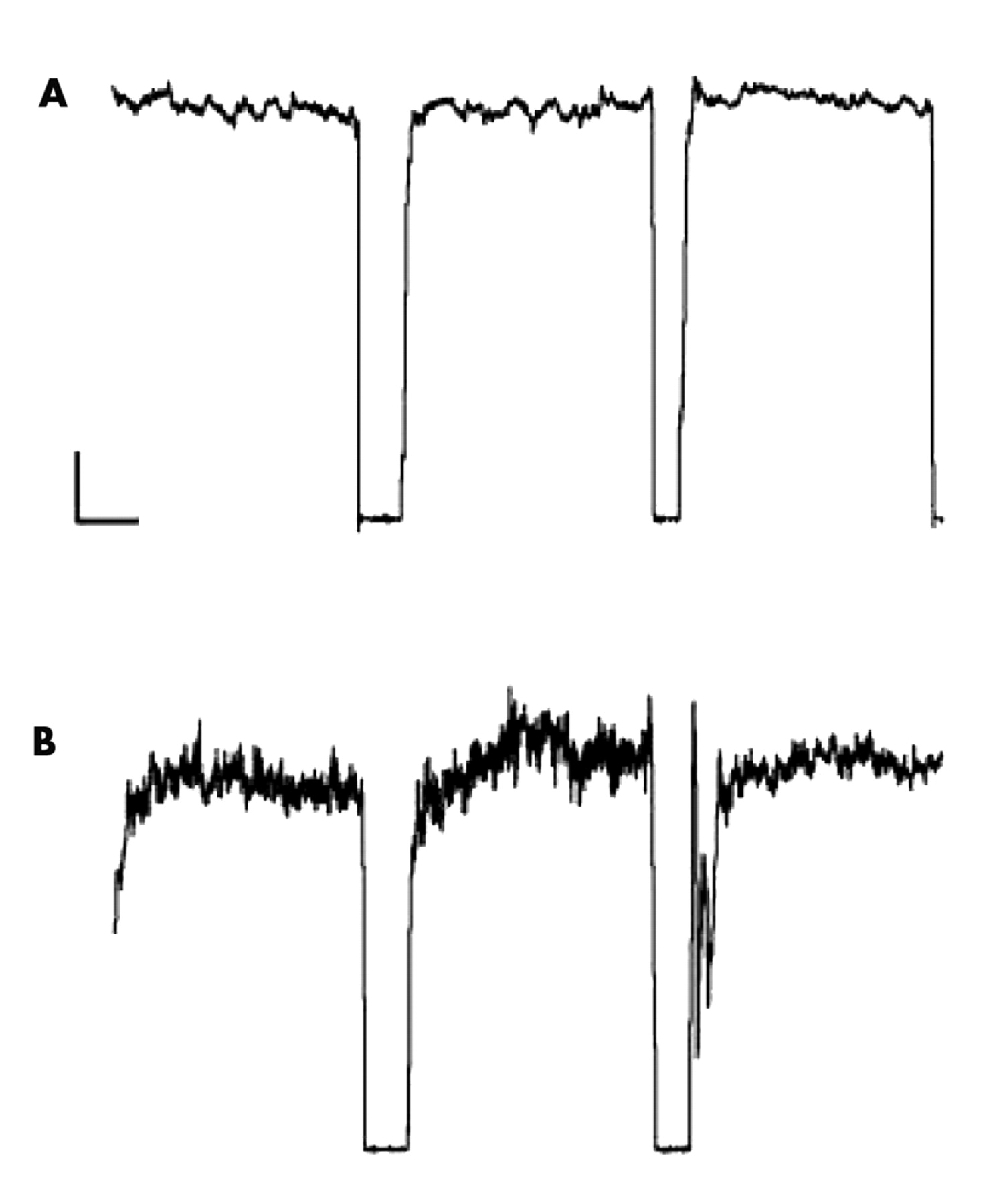
12 The two main methods we have developed assess the ability to maintain steady-state force and the ability to scale velocity with distance. Although we have observed that patients with clinically observable movement disorders show abnormalities on these measures (with abnormalities in force steadiness being seen in movement disorders marked by a hyperdopaminergic state, and velocity scaling abnormalities being associated more with a hypodopaminergic condition),
13 we also have found that patients without obvious clinical movement problems may demonstrate abnormalities on these measures. In particular, we have found that patients with schizophrenia may show abnormalities on these measures without evidence of frank tardive dyskinesia or parkinsonism.
14We hypothesized that patients with bipolar disorder would show abnormalities on these measures of force steadiness and velocity scaling. Further, because of the possible importance of hyperdopaminergia in mania
15,
16 and hypodopaminergia in depression,
17,
18 we considered that performance on the force steadiness and velocity scaling measures would correlate with symptoms of mania and depression, respectively. In particular, we hypothesized that the degree of manic symptoms would positively correlate with force steadiness scores, and the degree of depressive symptoms would negatively correlate with velocity scaling scores (lower velocity scaling scores indicate less impairment). A third objective of the study was to explore whether impaired motor performance might relate to current pharmacotherapy.
RESULTS
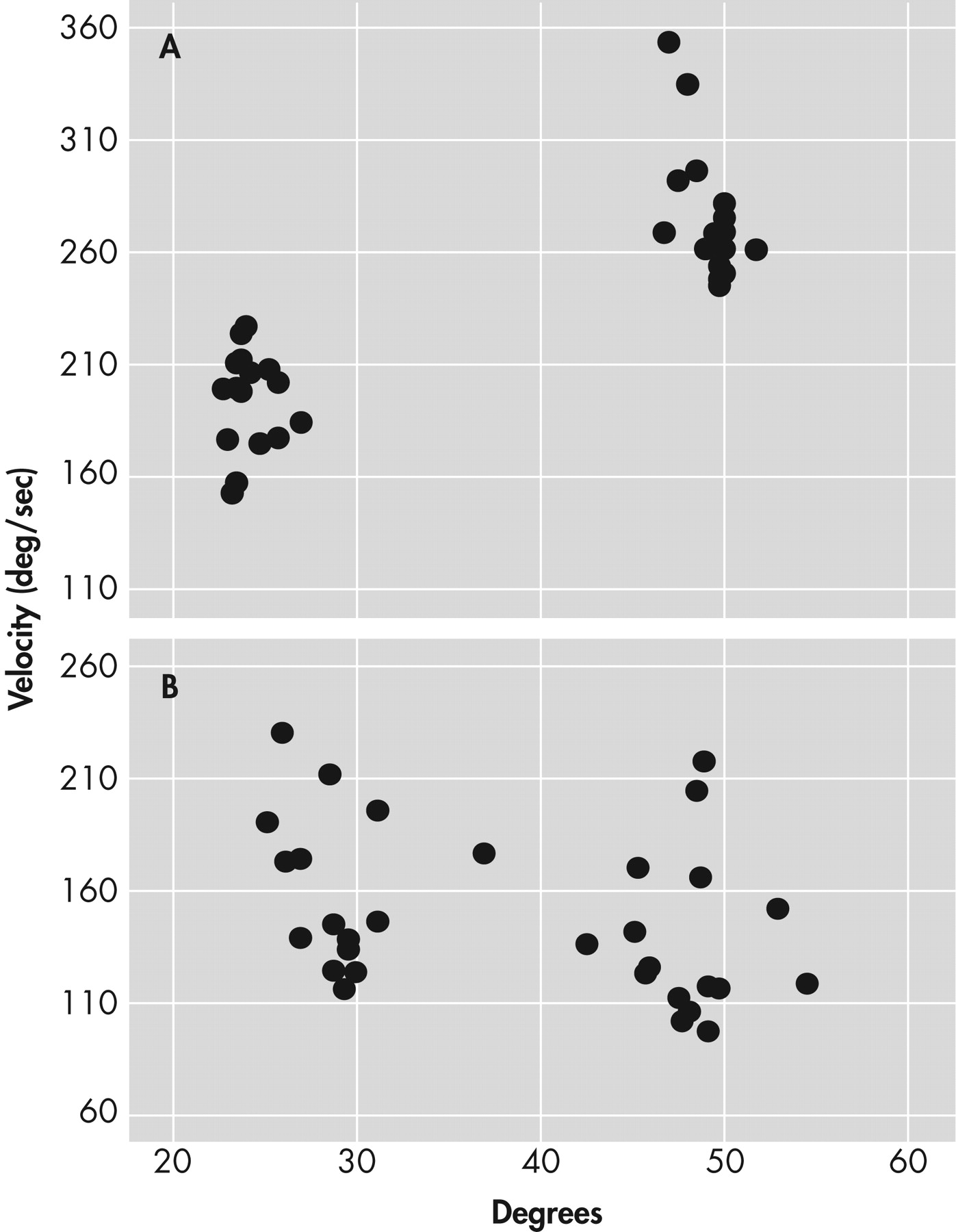
Overall, bipolar disorder patients performed significantly more poorly than healthy comparison subjects. The mean scores for the force steadiness and velocity scaling measures for the healthy comparison group were 1.96 (SD=0.77) and 2.53 (SD=1.35), respectively. The mean scores for the force steadiness and velocity scaling measures for the bipolar disorder patients were 3.46 (SD=3.00) and 1.71 (SD=0.92), respectively. Nonparametric tests for group differences revealed highly significant differences between bipolar disorder patients and healthy comparison subjects for the force steadiness (p<0.00001) and velocity scaling (p<0.001) measures.
For the force steadiness measure, 39 patients (58%) scored within the abnormal range. For the velocity scaling measure, 42 out of 67 patients (63%) scored within the abnormal range. Twenty-five bipolar disorder patients (37%) exhibited both force steadiness and velocity scaling abnormalities, and 56 patients (84%) had abnormal measures on either assessment. Scores on the force steadiness and velocity scaling measures were unrelated to each other. Also, the percentage of patients with abnormalities on both measures (37%) is no greater than would be calculated from a chance relationship between the two measures (58% x 63% = 36%).
Affective State
Patients with abnormal force steadiness scores did not differ from patients with normal force steadiness scores on ratings of depression or mania. Patients exhibiting both force steadiness and velocity scaling abnormalities did not differ from other subgroups on these ratings of mood pathology. There were no significant relationships between severity of mood symptoms based on the HAM-D and YMRS scores and the force steadiness or velocity scaling measures. The Pearson’s r values for the correlations between HAM-D and the motor measures were −0.19 (force steadiness) and −0.09 (velocity scaling), and values between YMRS and the motor measures were −0.06 (force steadiness) and 0.17 (velocity scaling).
Medication Effects
Because of the known influence of certain psychotropic medications on dyskinesia and parkinsonism, we examined whether the motor abnormalities described above might be related to current medication status. The bipolar disorder subjects were divided into those who were being treated with a particular class of psychotropic medications and those who were off these medications. Patients on current pharmacotherapy were separated into five groups, including subjects on antidepressants, mood stabilizers, antipsychotics, and benzodiazepines, and those off medication at the time of testing. We examined each medication group to determine if it was associated with a greater prevalence of instrumentally measured motor abnormalities. Prevalence results indicated no significant differences between the numbers of bipolar disorder subjects meeting criteria for force steadiness or velocity scaling impairment among any of the groups. Prevalence rates for medicated subjects ranged from 50% (for antipsychotics or benzodiazepines) to 62.5% (for antidepressants) for force steadiness impairment, and 50% (for benzodiazepines) to 80% (for antipsychotics) for velocity scaling impairment. Although there were only 11 unmedicated bipolar disorder subjects, seven of the 11 (63.6%) unmedicated bipolar disorder subjects exhibited abnormal force steadiness scores and five (53.5%) exhibited abnormal velocity scaling scores. Thus, the prevalence of instrumentally derived motor abnormalities among unmedicated bipolar disorder subjects was comparable to that of the medicated subjects.
DISCUSSION
We addressed three questions about motor pathophysiology in bipolar disorder. First, we examined whether bipolar disorder patients exhibit disturbances in motor physiology that may relate to abnormalities within the sub-cortical motor circuits. We found that approximately 60% of our bipolar disorder patients exhibited force steadiness or velocity scaling motor abnormalities based on instrumental assessments, with 84% of patients having abnormalities on either measure. Over one-third of the patients exhibited both forms of motor impairment. The second question of this study was to examine whether disturbances in motor physiology in bipolar disorder patients as assessed by force steadiness and velocity scaling were related to an affective state in bipolar disorder. We found no correlation between the motor scores and the severity of manic symptoms (as measured by the YMRS) or depressive symptoms (as measured by the HAM-D). The third question pertained to whether the observed impairment in motor functions was possibly related to current pharmacotherapy. We found no differences in prevalence of impairment between bipolar disorder patients on versus off psychotropic medications. Moreover, unmedicated bipolar disorder patients exhibited similar distributions of force steadiness and velocity scaling impairment as treated patients. The possibility remains that past exposure to certain medications could have contributed to the observed abnormalities in motor function.
The lack of associations between mood state, pharmacotherapy, and force steadiness or velocity scaling performance indicates that these motor impairments may be trait characteristics of bipolar disorder. However, caution is warranted in this interpretation because the present study was cross-sectional and not longitudinal in nature. Nevertheless, it is possible that these measures may be useful as endophenotypes in future genetic studies of bipolar disorder.
With the exception of psychomotor retardation
34 or lithium-induced tremor,
35 few studies have focused specifically on motor abnormalities in bipolar disorder. The few exceptions include studies by Heninger and Kirstein,
36 who reported increased motor activity in manic bipolar disorder patients, and Klein et al.,
37 who reported that increased motor activity predicted relapse into mania in bipolar patients. It may be argued that this lack of attention to motor disturbances in bipolar disorder may be related to their relative infrequency in bipolar disorder in comparison to schizophrenia
38,
39 or major depression.
34 Motor problems in bipolar disorder may also be underreported because they can be subtle and difficult to assess using conventional assessment means.
40Our findings show that approximately 60% of patients with bipolar disorder demonstrate significant impairments in the ability to maintain steady-state force or the ability to scale velocity with distance; but these two findings appear to be independent, suggesting that two separate physiological mechanisms relating to basal ganglia circuitry may be involved in bipolar disorder. In fact, the putative neural circuits involved in motor dysfunction have been elucidated over the past two decades
41,
42 and two basic pathways have been identified. Neurotransmission from the motor cortex, through the basal ganglia and thalamus, and back to the sensorimotor cortices has been proposed to follow a direct pathway and an indirect pathway, the latter of which involves the subthalamus.
43 Disturbances within the direct pathway have been suggested to be associated with the reduction of movement or with difficulty initiating movement (hypokinesia), whereas disturbances within the indirect pathway have been proposed to be associated with excessive or unstable movement (hyperkinesia). In this context, our results suggest that velocity scaling abnormalities may possibly relate to dysfunction in the direct pathway, and the force steadiness abnormalities to disturbances in the indirect pathway in bipolar disorder. Although this is highly speculative, these brain regions could serve as a focus in future studies utilizing techniques such as functional magnetic resonance imaging (fMRI).
Our findings suggest that psychopathology and motor dysfunction may share a common pathophysiology. Comorbid mood and motor disorder are common features in neuropsychiatric disorders, such as Parkinson’s and Huntington’s diseases. For example, depression is common among patients with Parkinson’s disease with prevalence estimates ranging from 20% to 45%.
43,
44 Studies have shown that the onset of depressive symptoms often predates the presentation of motor signs in Parkinson’s disease.
45 This would imply that the mood symptoms may be more closely linked to the disease process than a secondary feature of Parkinson’s disease. Based on a study using positron emission tomography (PET), Remy et al.
46 suggested that depression in Parkinson’s disease might be associated with reduction in dopamine and norepinephrine receptor binding in structures including limbic regions.
Both depression and mania are observed in Huntington’s disease, a prototypic movement disorder of basal ganglia origin. Estimates of the prevalence of a mood disorder in Huntington’s disease range from 22% to 38% for depression
47 and 5% to 10% for mania.
48 PET studies in depressed Huntington’s disease subjects also show pathophysiological correlates (e.g., glucose hypometabolism in the orbitofrontal and inferior parietal regions of the brain) that are similar to those observed in major depression.
49The co-occurrence of mood symptoms with parkinsonism and choreoathetosis in neurological diseases supports the hypothesis that hypokinetic or hyperkinetic motor abnormalities may coexist with affective disorders because of shared pathophysiology. The present findings of motor problems in bipolar disorder suggest an extension of this hypothesis to include psychiatric disorders as well. Although we did not observe a significant relationship between the severity of mania or depression and severity of motor dysfunction in this cross-sectional study, a longitudinal study might reveal that a fluctuation in affective state is related to a fluctuation in motor control.
In conclusion, the results from this study contribute to the literature by characterizing motor abnormalities in bipolar patients. The use of sensitive quantitative instrumentation of motor function may be helpful in characterizing the brain abnormalities of bipolar disorder. Moreover, better characterization of the motor circuits underlying these abnormalities may help us determine which circuits are disrupted in the modulation of affect and where to target novel treatments.