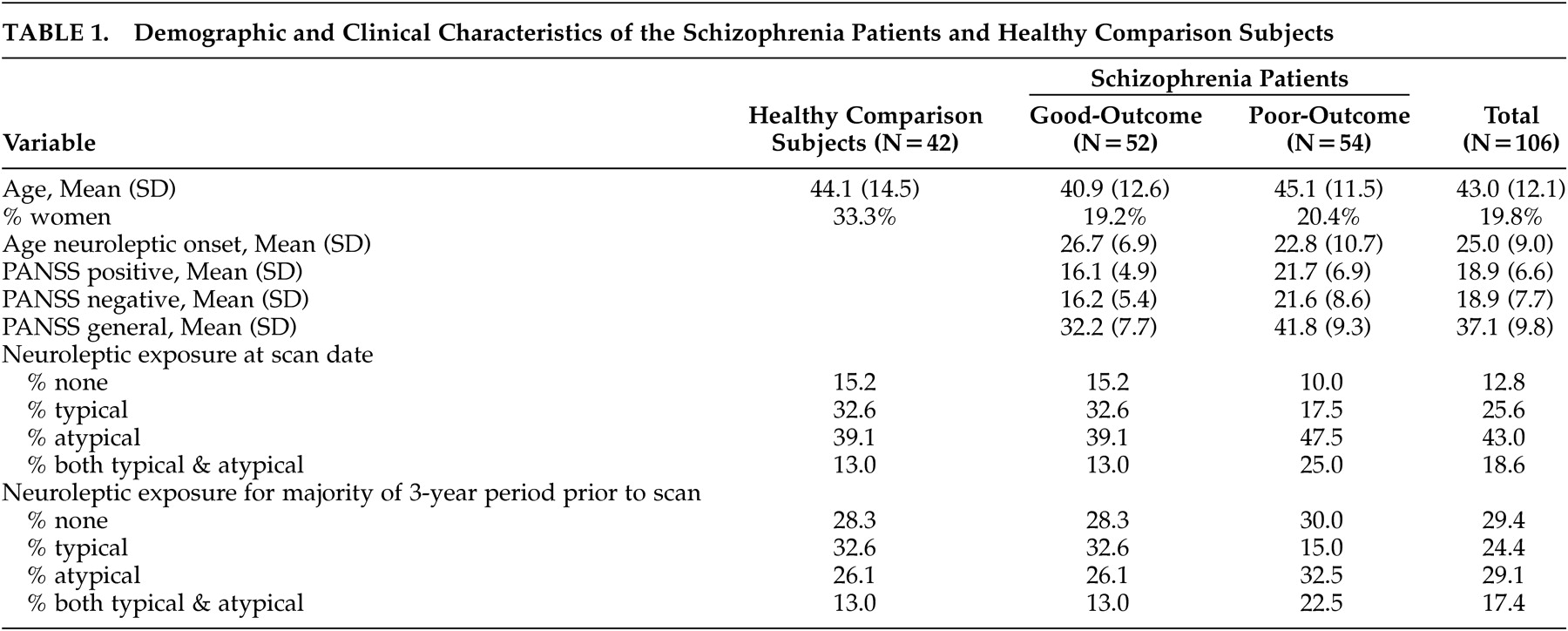
T here has been a recent focus on defective white matter as central to the pathophysiology of schizophrenia following a culmination of genetic, imaging, and post-mortem evidence of oligondendroglia or myelin-related abnormalities.
1 These deficits may affect white matter throughout the brain or be restricted to specific neural circuits. Structural MRI studies that have examined white matter volume as a whole have been somewhat equivocal, perhaps because white matter volume reduction in schizophrenia is reported to be of small effect size. For example, a recent meta-analysis demonstrated only a 1% total white matter volume loss compared to healthy subjects.
2 Specific regional investigations of white matter in frontal
3 –
7 and temporal areas
8,
9 have been more consistent in their findings of schizophrenia-related volume reduction.
A problem with several studies of white matter in schizophrenia is that they have examined gross regional areas without measurement of specific pathways that are implicated in schizophrenia-associated circuitry abnormalities. This limitation is likely a result of the inherent difficulty in the quantification of white matter, given its diffuse representation throughout the entire brain. Although few white matter pathways have discrete anatomical borders, the anterior limb of the internal capsule is uniquely delimited by subcortical nuclei that can be used for reliable anatomical landmarks in manual tracing or region-of-interest (ROI) approaches. The anterior limb of the internal capsule contains descending motor and ascending sensory fibers interconnecting brain regions implicated in the pathophysiology of schizophrenia, including neocortex, striatum, thalamus, and pons. Disruption of this fiber bundle can result in cognitive deficit with a “subcortical” profile that is similar to the cognitive deficits seen in schizophrenia.
10 Furthermore, the internal capsule contains a heterogeneous set of neurons, including cholinergic and noncholinergic projections, which may mediate sensory and behavioral functioning often affected in schizophrenia.
11 The anterior limb of the internal capsule contains frontothalamic and thalamofrontal pathways, the corticopontine pathways, and a lesser number of caudate/pallidum fibers.
12,
13 Thalamocortical fibers appear most concentrated in the anterior tip, while the posterior section of the anterior limb of the internal capsule has primarily corticopontine fibers.
13 Reduction in the size of the internal capsules in patients with schizophrenia would be consistent with diminished corticothalamic and corticostriatal connectivity and with previous reports of schizophrenia-associated abnormalities in the striatum
14,
15 and thalamus.
16 –
21 Indeed, a recent study from our laboratory
22 demonstrated a significant relationship between frontal lobe size and thalamus size in schizophrenia patients with poor functional outcome, suggesting faulty interconnections between the two structures.
Previous studies examining the morphology of the internal capsules in schizophrenia suggest a reduction in volume. Manual tracings of the anterior limb of the internal capsules on coronal magnetic resonance imaging (MRI) slices in 53 patients with schizophrenia and 48 healthy comparison subjects revealed bilateral decrease in volume in the patient group.
23 A similar study of patients with schizotypal personality disorder, thought to be on the schizophrenia “spectrum,” reported volume reduction in the right internal capsule.
24 Schizophrenia-associated volume decreases in the internal capsules were also observed with voxel-based morph(ometry,
23 –
25 although one study using voxel-based morphometry did not find anterior limb volume differences between patients with schizophrenia and healthy comparison subjects.
5A study of 39 schizophrenia patients and estimates from voxel-based morphometry showed significant correlations between right posterior internal capsule volume and illness duration, but contrasts with normal volunteers were not available.
26 Multiplanar manual tracing of the internal capsule can provide better regional specificity than approaches that consider voxel-wide or full volume comparisons, which require spatial normalization that might obscure the shape of smaller regions (see Giuliani et al.
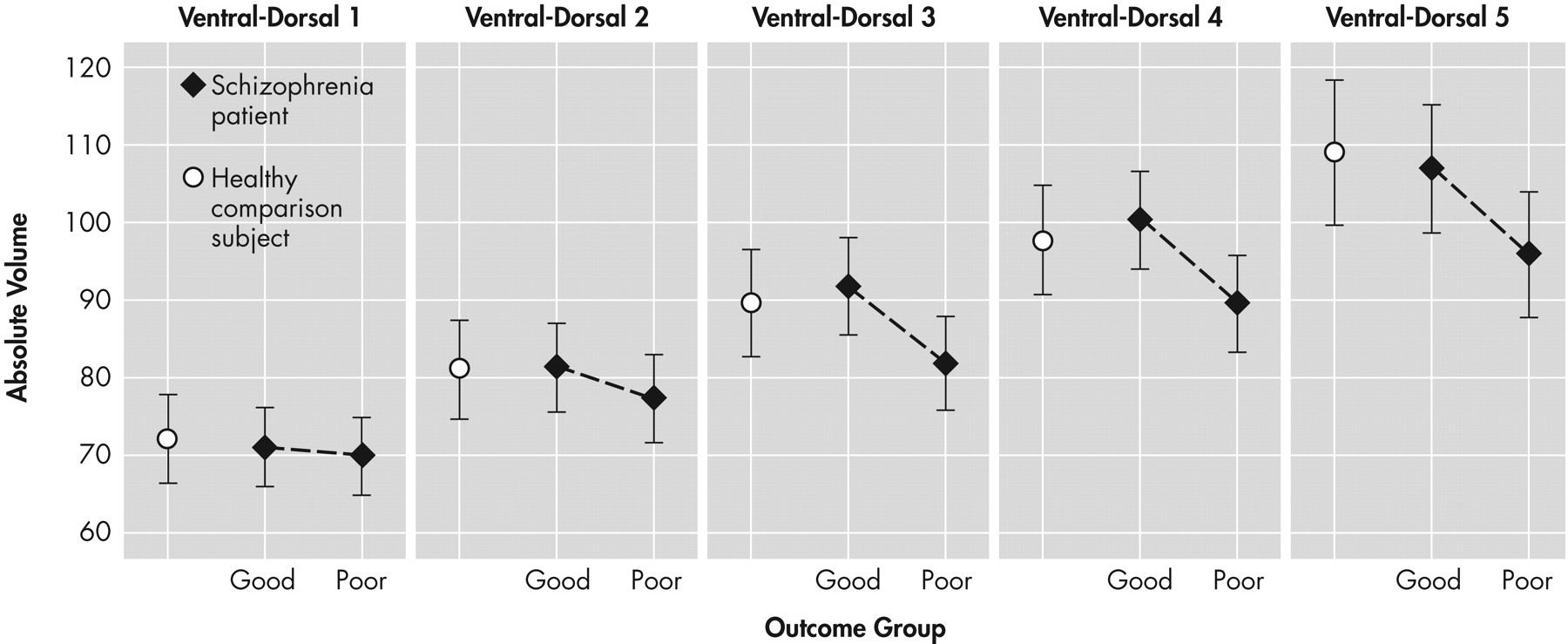
27 for discussion). By tracing the internal capsules at various slices in the dorsal-to-ventral plane, comparisons with other brain regions at various slice levels can be made to examine more comprehensively the interrelationships among brain regions. Furthermore, multiplanar tracing allows for examination of the shape of the internal capsules as well as overall size. For example, the lateral ventricles appear at more dorsal levels of the internal capsules, but not at ventral levels. Expansion of the lateral ventricles could put pressure on adjacent internal capsule fibers, thus narrowing the fiber tract at dorsal levels or pushing them in the ventral direction without affecting the overall size or volume of the structure.
Given previous findings of ventriculomegaly in poor-outcome patients,
28,
29 it is important to explore the relationships between the size of the internal capsule at different levels and the size of the lateral ventricles. Further, examination of the intercorrelation among the volumes of brain regions may shed light on potential trophic relationships or circuitry abnormalities implicated in schizophrenia (see Mitelman et al.
30 for discussion). Thus, another goal of the current study was to explore the relationship between internal capsule size and volumes of other regions implicated in good and poor-outcome schizophrenia.
16,
31 –
33 For the current study, a novel neuromorphometric region-of-interest protocol for examining the size of the anterior limb of the internal capsule was developed. The primary purpose of the study was to examine the size of the anterior limb of the internal capsule in healthy comparison subjects compared to good- and poor-outcome schizophrenia patients (“non-Kraepelinian” and “Kraepelinian” patients, respectively). Distinguishing patients by functional status has been one strategy of reducing clinical heterogeneity in schizophrenia. Poor-outcome patients are defined as being dependent on others for food, clothing, and shelter for a continuous period of 5 years or longer, whereas good-outcome patients are more independent in these functional domains.
34 Recent MRI studies have shown reliable differences between the two groups in cortical gray matter distribution,
32 cingulate cortex volume,
33 striatal size,
31 and thalamus size.
16,
22 We hypothesized that there would be a reduction in internal capsule size in the two schizophrenia groups, which would be more pronounced in the poor-outcome patients. Further, we expected that the size of the internal capsules would be related to the severity of psychopathology and to volumes of surrounding cortical and subcortical regions.
DISCUSSION
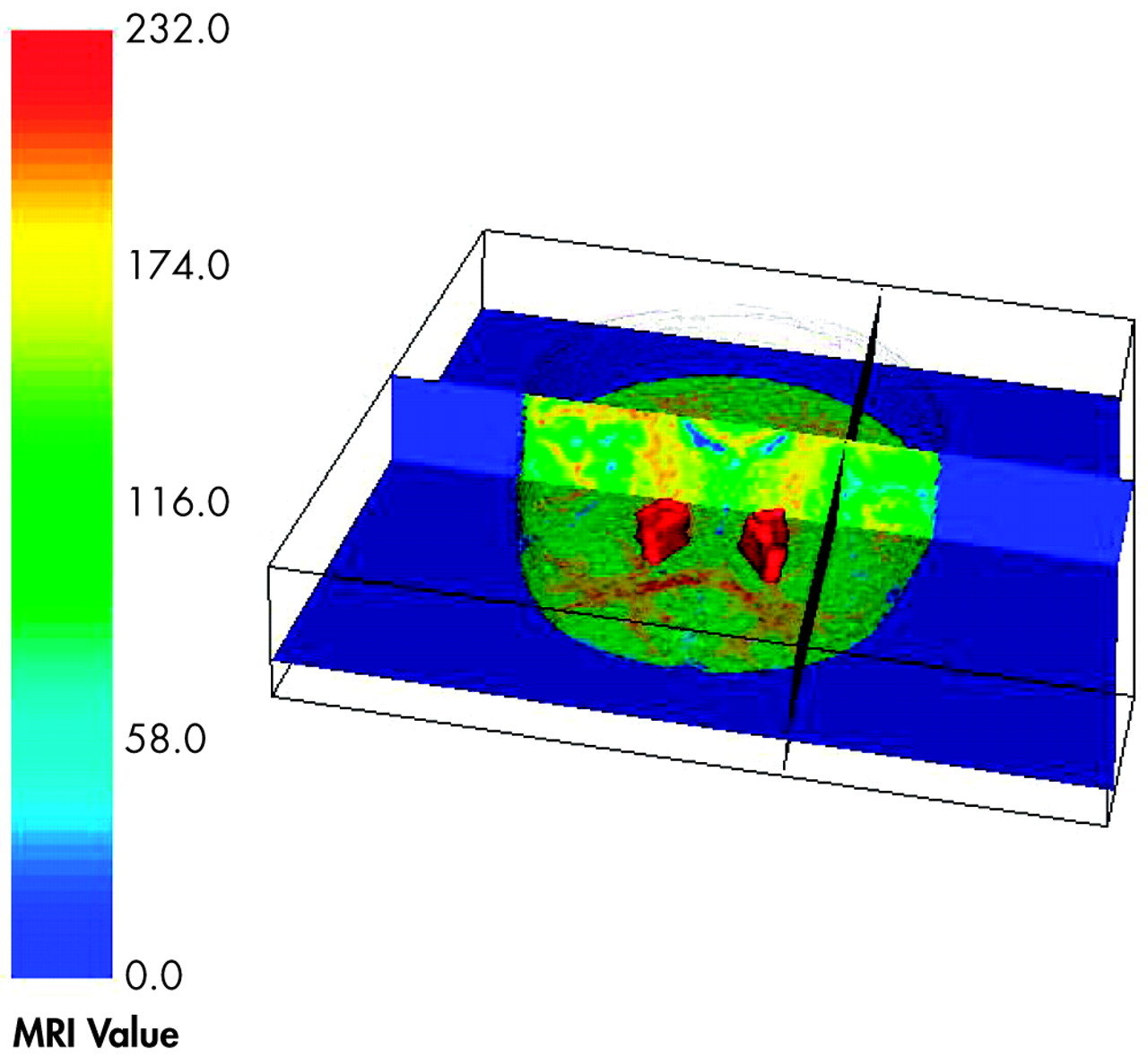
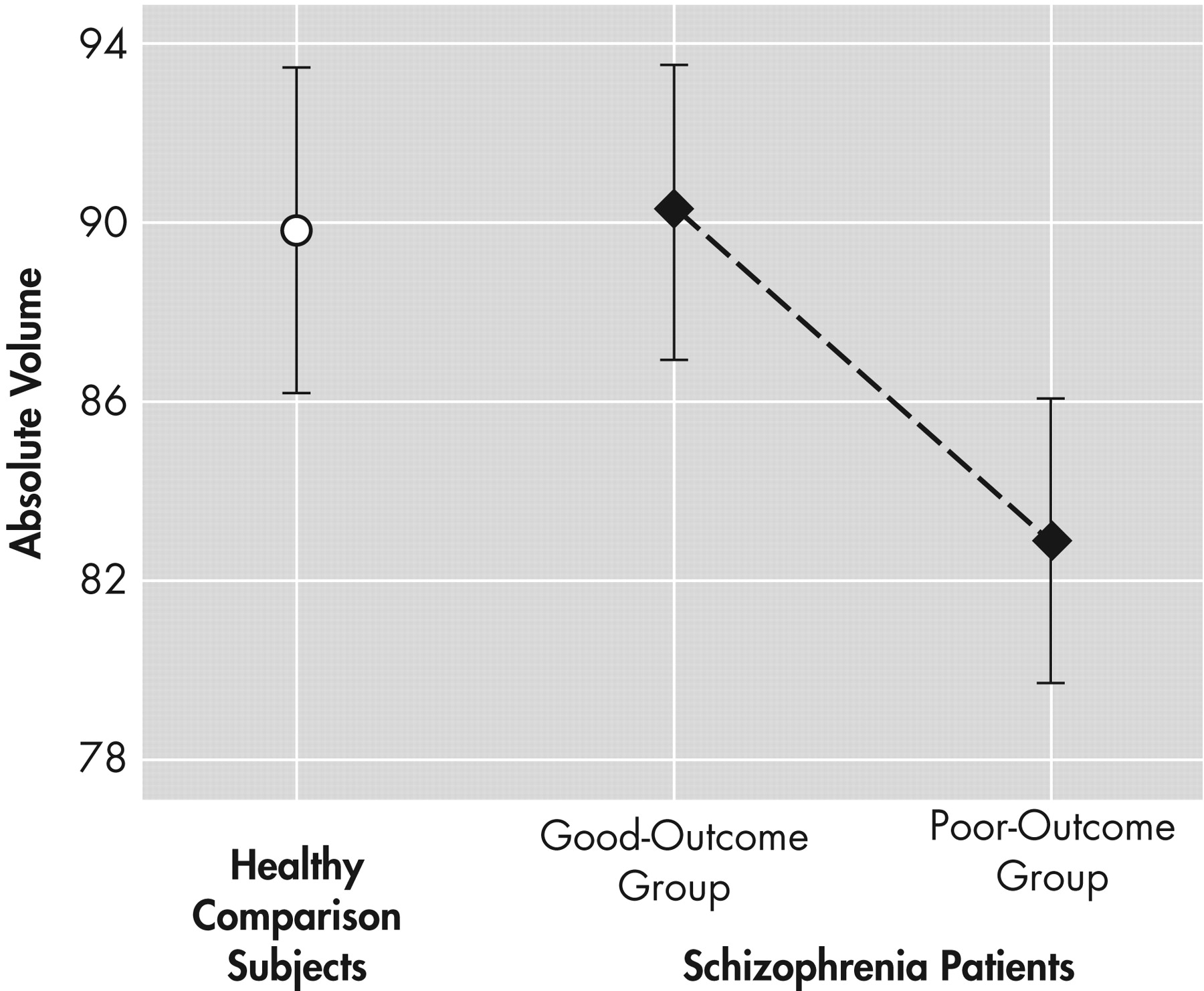
The findings from this study provide tentative support for internal capsule size reduction in schizophrenia. These abnormalities may be restricted to dorsal levels of the internal capsule in poor functioning patients and are associated with age. Significant correlations emerged, particularly between internal capsule size in healthy comparison subjects and the surrounding size of subcortical brain regions. However, these associations tended to become weaker with progressive decline in functional outcome. Taken together, these findings are suggestive of internal capsule abnormalities in poor-outcome patients, which may reflect a neurodegenerative process and disrupted cortical-subcortical circuitry. Analyses of relative and absolute internal capsule size revealed several important findings. First, schizophrenia patients, as a single group, did not significantly differ from healthy comparison subjects. A trend level interaction on relative values suggested that poor-outcome patients had smaller internal capsules at more dorsal levels and larger internal capsules at more ventral levels than both good-outcome patients and healthy comparison subjects. Analyses on absolute values confirmed that poor-outcome patients had significantly smaller internal capsules, particularly at dorsal levels. Taken together, the findings suggest abnormal fiber distribution in poor-outcome patients. Only one published study, to our knowledge, has taken an a priori, region-of-interest approach to examining the internal capsules in schizophrenia.
23 In that study, the authors used manual tracing and confirmatory analysis with voxel-based morphometry to demonstrate that total bilateral anterior limb volume was decreased in schizophrenia patients compared to matched healthy subjects. Findings from the current study are somewhat consistent with this report; reduced internal capsule size was found, but restricted to poor-outcome patients. Discrepancies between the two studies could be due to subject characteristics. That is, functional status (e.g., inpatient, outpatient, or institutionalized) and disease severity of the patient sample in the Zhou et al.
23 paper were not reported. Thus, their sample could have been composed of particularly poor functioning patients. Unlike Zhou et al., the current study did not find greater right- than left-sided asymmetry in the patient group relative to healthy comparison subjects.
Two other studies using voxel-based morphometry have reported significant anterior limb internal capsule reduction in schizophrenia patients compared to healthy subjects.
24,
25 These voxel-based morphometry studies are somewhat exploratory because they did not test a priori hypotheses about specific anatomical regions. Instead, they provided important information on a whole brain, voxel-by-voxel basis. Thus, the two positive findings of internal capsule decreases in schizophrenia with this method should be interpreted cautiously given that most studies using voxel-based morphometry have failed to report similar differences. Because the internal capsules comprise a portion of the larger corona radiata, which has widespread cerebral distribution, their fibers are at least indirectly involved with projections to and from most cortical regions.
39 In terms of the specific pathways comprising the region, the anterior thalamic peduncle forms a large portion of the anterior limb, connecting anterior and medial nuclei of the thalamus with the cingulate gyrus and with dorsolateral and medial aspects of the frontal lobe, as has been demonstrated with polarized light and confocal scanning laser microscopy.
13 Fibers extending between caudate nucleus and globus pallidus are also found in the anterior limb,
12,
13 particularly at dorsal levels of the pallidum.
40 Finally, frontopontine fibers, which were well described in classic post-mortem studies of individuals with leucotomy,
41 have been shown to occupy approximately 37% of anterior limb volume.
12 Consistent with the systematic distribution of the corona radiata throughout the brain, fibers coursing through the anterior limb of the internal capsule are topographically organized,
39 particularly in the anterior-posterior direction.
12 However, several of the fiber bundles intertwine with one another and are distinguishable by their slope of projection, rather than their discrete boundaries.
13 Consideration of the pathways contained within the anterior limb of the internal capsules suggests particular frontothalamic, thalamofrontal, and intrastriatal abnormalities in poor-outcome patients. Findings from our recent examination of the thalamus,
16 indeed, demonstrated thalamic volume reduction in the same cohort of poor-outcome patients compared to good-outcome patients and healthy comparison subjects. Furthermore, the results are consistent with a study on a subsample of the included participants,
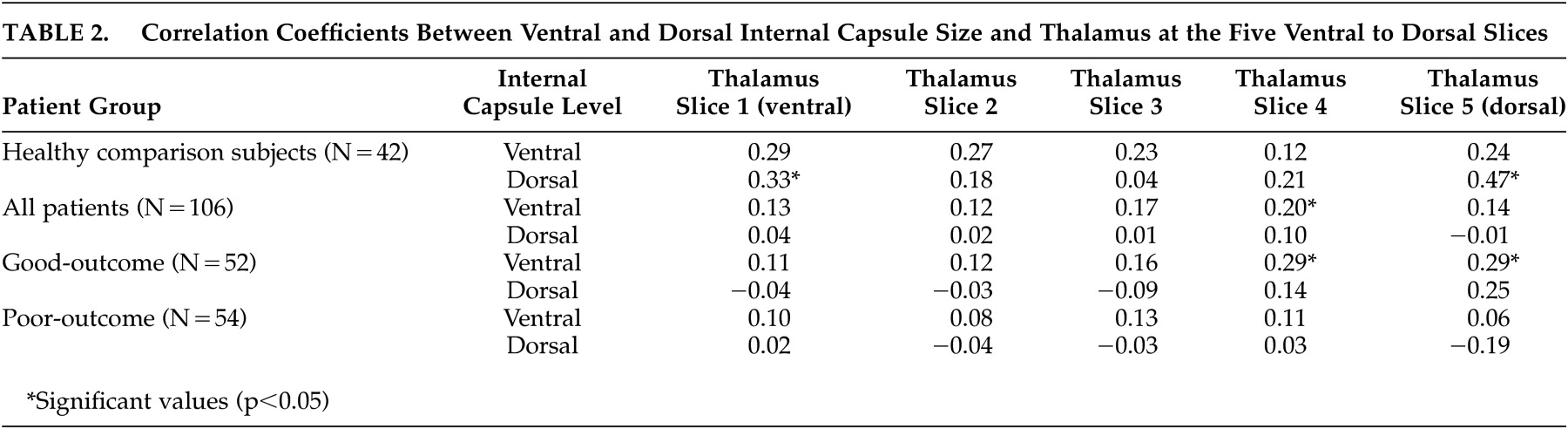
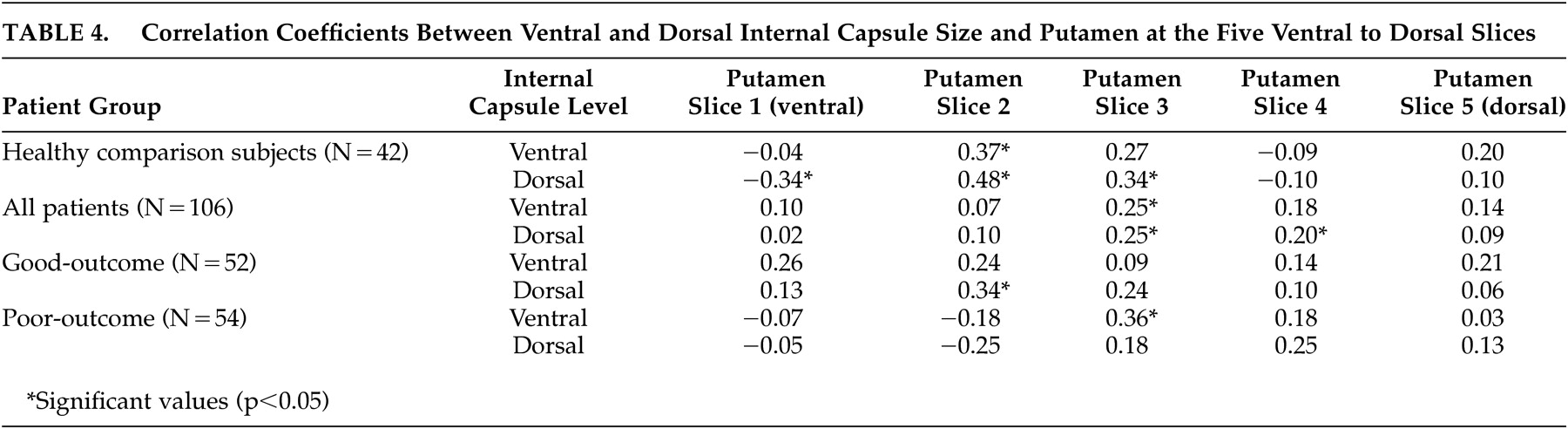
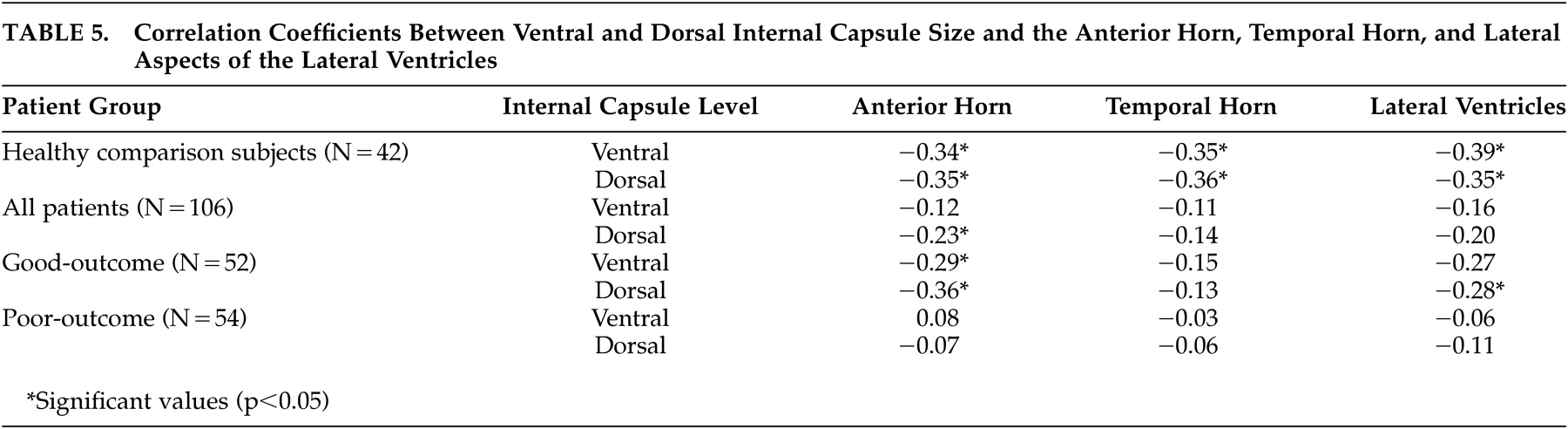
31 which suggested striatal abnormalities in poor-outcome patients. Correlation coefficients examining the relationship between internal capsule size and the size of surrounding cortical and subcortical regions provide some further insight into the nature of the internal capsule size reduction seen in the poor-outcome patients. By far, the most consistent associations were between ventral and dorsal internal capsule size and all aspects of the lateral ventricles; larger ventricles were associated with smaller internal capsules in healthy comparison subjects.
This normal relationship suggests that enlargement of the ventricles contributes to reduction in size of the internal capsule, potentially as a secondary effect of atrophy or increased ventricular pressure. However, this association was not as consistent in the good-outcome patients and nonexistent in the poor-outcome patients. The markedly enlarged ventricles
28,
42 and reduced internal capsule size in the poor-outcome patients may be, therefore, the result of independent pathophysiological processes. This finding lends support to the idea that ventricular enlargement in poor-outcome patients is endophenotypic and does not cause secondary surrounding structural reduction.
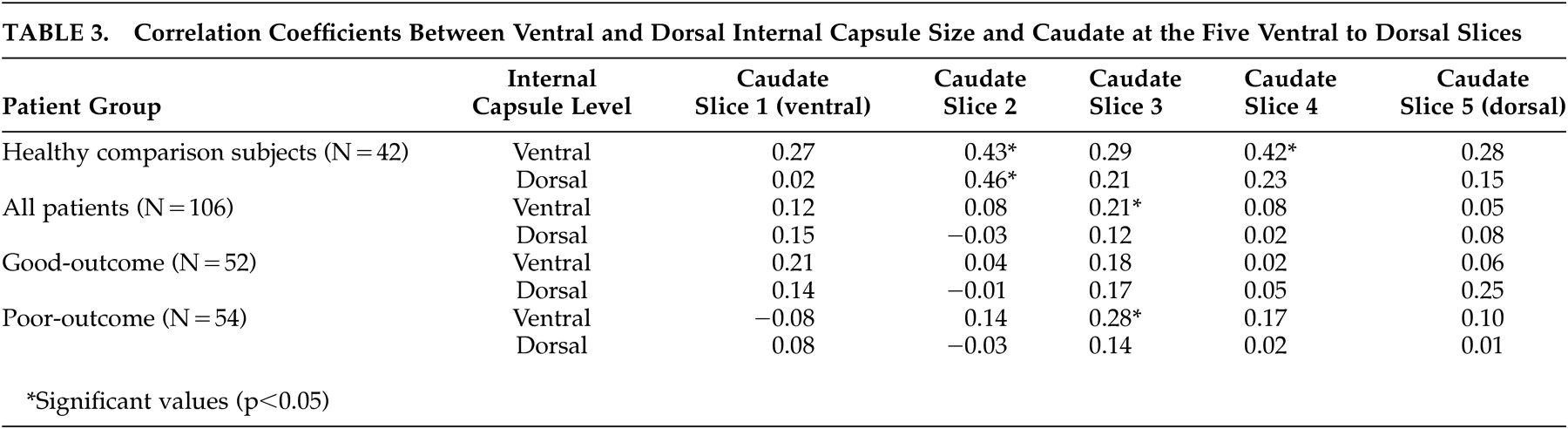
Consistent with the report by Zhou et al.,
23 we found significant positive associations between ventral and dorsal internal capsule size and some levels of the caudate in the healthy comparison subjects, but these associations were less consistent in either of the patient groups. Dorsal internal capsule sizes were not related at all to caudate size at any level in either of the schizophrenia groups when considered separately or alone. Given that the caudate nucleus forms the medial border of the internal capsules, the finding of a normal association between the two structures is not surprising. However, the lack of association in the schizophrenia patients, particularly the poor-outcome group, suggests that internal capsule size reduction is independent of variability in the caudate. Our findings are not consistent with those of Hulshoff Pol et al.,
25 who reported negative associations between internal capsule and caudate density, attributing the finding to normal brain maturation.
A somewhat similar pattern emerged for associations with the putamen. Significant correlations were strongest in healthy comparison subjects, but still present in both schizophrenia groups. Given that slices for analysis of the internal capsule were chosen based on putamen morphology and that the internal capsule borders the lenticular nucleus, these associations are not unexpected. Although some significant positive associations were found between ventral and dorsal aspects of the internal capsule and levels of the thalamus in all three groups, they were less consistent than expected. Dorsal internal capsule was associated with dorsal and ventral thalamic size in healthy comparison subjects, whereas ventral aspects of the internal capsule were associated with dorsal slices of the thalamus in good-outcome patients. Poor-outcome patients evidenced no significant relationships. These findings tentatively suggest a greater disruption in circuitry as a function of worsening functional outcome, but do not support the idea that one structure affects the other. Similarly, there was a lack of significant associations between internal capsule size and cortical gray or white matter volume in any of the groups.
The current study found a significant negative association between age at the time of scan and dorsal internal capsule size in the poor-outcome group, but not in the good-outcome patients. The finding speaks to the possibility that poor-outcome patients, who exhibited dorsal internal capsule size reduction, have a progressive, neurodegenerative course. It is also somewhat consistent with a report by Velakoulis et al.,
26 who found a negative association between right internal capsule volume and illness duration in a group of schizophrenia patients. Similar to Hulshoff Pol et al.,
25 the current study found little relationship between internal capsule size and exposure to neuroleptic treatment and only a modest effect of sex on internal capsule size.
There are some limitations to the current study. The anterior limb of the internal capsules contains fibers that are part of the corona radiata, a much larger, diffusely distributed cortical-subcortical white matter system. A benefit to studying morphometry of the internal capsules is that it is largely contained within surrounding subcortical structures, facilitating reliable boundary demarcation. However, positive findings in the internal capsule are likely to be representative of much more diffuse white matter changes; given the intertwining fiber bundles and the anterior-posterior topography contained within the structure,
13 it is difficult to draw conclusions about which of the specific pathways contained within the internal capsules are implicated, particularly when considering the internal capsules in the dorsal-ventral direction. Studies using diffusion tensor, an MRI sequence that provides information about the coherence and directionality of white matter fibers, would complement morphometric efforts and help better characterize white matter abnormalities in good- and poor-outcome patients. Indeed, in the first study using diffusion tensor in schizophrenia patients, Buchsbaum et al.
43 demonstrated anisotropic reductions in areas of the anterior limb of the internal capsule. Further, preliminary diffusion tensor analyses with a subset of patients from the current cohort
44 showed schizophrenia-associated anterior anisotropic reduction, but greater reduction in posterior regions in poor-outcome patients. A second limitation is the restriction of the area to definition by four points to enhance reliability, although the geometry of the parallelogram shape is generally well matched by the internal capsule appearance.
In conclusion, internal capsule size reduction in this study was restricted to poor-outcome patients at dorsal levels and appeared to be somewhat related to age. These findings suggest neurodegeneration in this poor-outcome group. Internal capsule size appeared to be most related to ventricular size in healthy comparison subjects, but this relationship was less strong in good-outcome patients and nonexistent in poor-outcome patients. Disruption of fibers coursing through the internal capsule in poor-outcome patients may be reflective of myelin-related or oligodendroglial abnormalities or neurodevelopmental maturational problems. Findings from this study are less supportive of trophic effects.