P ostoperative cognitive dysfunction is defined as a deterioration of intellectual function characterized by impairment of memory and concentration after surgical procedures.
1 The existence of prolonged cognitive dysfunction postoperatively has been disputed, but an international cooperative research group found at least 1% of elderly surgical patients to be significantly impaired 1 to 2 years after their surgical procedure.
2 Most people in countries with reasonable health services have experienced at least one surgical procedure involving a general anesthetic during their lifetime. Since publication of the article by Bedford,
3 it remains a matter of controversy whether the surgical procedure and its complications alone or the exposure to general anesthesia, or both, explains postoperative cognitive dysfunction. At least some studies favor the hypothesis that exposure to general anesthesia can cause even Alzheimer’s disease.
4 –
7 The influence of general anesthesia on cognitive functioning in elderly people has never been examined in a community-based study. We investigated whether the number of anesthesias during a lifetime influences cognitive functioning in elderly people in the community-based Vienna Transdanube Aging (VITA) study. We compare the effect of general anesthesia with the effects of proven risk factors for cognitive decline in elderly people.
METHOD
The procedure followed was in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki, Finland, Declaration of 1975, as revised in 1983. The research protocol was approved by the relevant ethics committee. Subjects were informed about the specific procedure and gave their written informed consent. The study design and recruitment procedure of the VITA has already been described.
8The VITA study is a community-based cohort study of all inhabitants of the 21st and 22nd districts of Vienna, Austria, who were born between May of 1925 and June of 1926. In a personal letter, the subjects were invited to participate in the cross-sectional investigation at the beginning of the study. Between May of 2000 and November of 2002, 606 subjects were examined completely. The participation rate was 46%. The investigation of participants was divided into a 90-minute-long screening assessment and a clinical investigation in the Danube Hospital with a duration of about 7 hours, including magnetic resonance imaging (MRI) of the brain in every subject.
We also tried to collect information on artifacts due to sample attrition. A consecutive series of nonrespondents (52 out of 75 who refused to participate) accepted a telephone interview concerning their current medication, which could be compared with the medication of VITA participants.
In order to get the most precise information about the number of surgical procedures under general anesthesia, we used a standardized interview about past and present disorders and possible surgical procedures structured by the various parts of the human body. Whenever possible, the subjects were asked these questions together with a close relative, and information regarding surgical procedures and anesthesias was corroborated by hospital charts and/or medical records. Only general anesthesias with complete loss of consciousness for more than 10 minutes were taken into account. The exact number of general anesthesias could be obtained for 603 out of the 606 subjects. We could not collect reliable data about the duration of anesthesias and about all surgical procedures that had taken place in the particular subjects because cognitive disturbances interfere with the memory of these details, especially in cases without a close informant. A history of major head trauma that had led to more than a 10-minute loss of consciousness was also derived from this interview. Education was measured as completed years of education. The psychosocial interview included questions about rural versus urban environments during childhood.
An MRI was possible for 532 out of 606 subjects. We rated atrophy of the medial temporal lobe according to the system used by Scheltens et al.
9 The cognitive domain was measured psychometrically by two clinical psychologists (SJ and SW) experienced in geriatric psychology. The complete battery of the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD)
10 was applied using the standardized German translation.
11 The CERAD battery includes the subtests Verbal Fluency, the Boston Naming Test, the Mini-Mental State Examination (MMSE),
12 Word List Memory (no distracter between item presentation and recall), Constructional Praxis, Word List Recall (delayed recall after distracter), Word List Recognition, and Constructional Recall. Dementia was rated according to the Clinical Dementia Rating.
13 Further psychometric tests used were the Fuld Object Memory Evaluation
14 and the Trail Making Test Part B (TMT-B),
15 a speed-dependent concept-shifting task which has been shown to describe postoperative cognitive dysfunction.
16 Results of the TMT-B represent time in seconds needed to complete the task, which means that higher scores indicate lower performance. Severity of depression was measured by the Short Geriatric Depression Scale, a self-rating instrument of 15 questions.
17The statistical analysis was performed by the SPSS-PC package, version 11.5, licensed by the University of Vienna. The Pearson correlation was used to assess associations between various cross-sectional measurements. Linear regression analyses were calculated with every test score as the dependent variable and a) years of education, b) childhood in an urban or rural area, c) atrophy of the medial temporal lobe, d) history of major head trauma, e) short geriatric depression rating, f) number of general anesthesias, g) body mass index, and h) gender. Independent predictors a, b, c, d, and e have already been proven as risk factors for cognitive decline in elderly people.
8,
18 –
23 The inclusion of a positive history of major head trauma into a regression analysis eliminated the possibility that general anesthesias associated with major head traumas are interpreted as the direct cause of cognitive dysfunction that originated in the head trauma itself. Fifty-one subjects (8.4%) had a positive history of at least one major head trauma. Regression coefficients (beta) and corresponding p values were calculated to describe the influence of a particular independent variable on cognitive status. The impact of predictors on the particular psychometric variable was described by means of R
2 . Graphic expression of residuals was performed to look for asymmetries in the distribution of residuals.
RESULTS
The VITA participants did not differ from the overall population with regard to gender, education, and socioeconomic status. We also carried out telephone interviews with 52 nonrespondents from three consecutive invitation rounds whose telephone numbers were available. In these interviews, we asked for current medications taken over the past 2 weeks and compared the data with the medications of VITA participants. There were no statistically significant differences concerning 30 types of medications. Overall, nonrespondents took fewer medications with regard to nootropics and cognitive enhancing drugs (piracetam, ergotamine, vitamin E, gingko biloba, pentoxifylline). They took other internal medications like antihypertensive drugs with nearly identical frequency. Participants took benzodiazepines (at lower dosage) less frequently than nonrespondents.
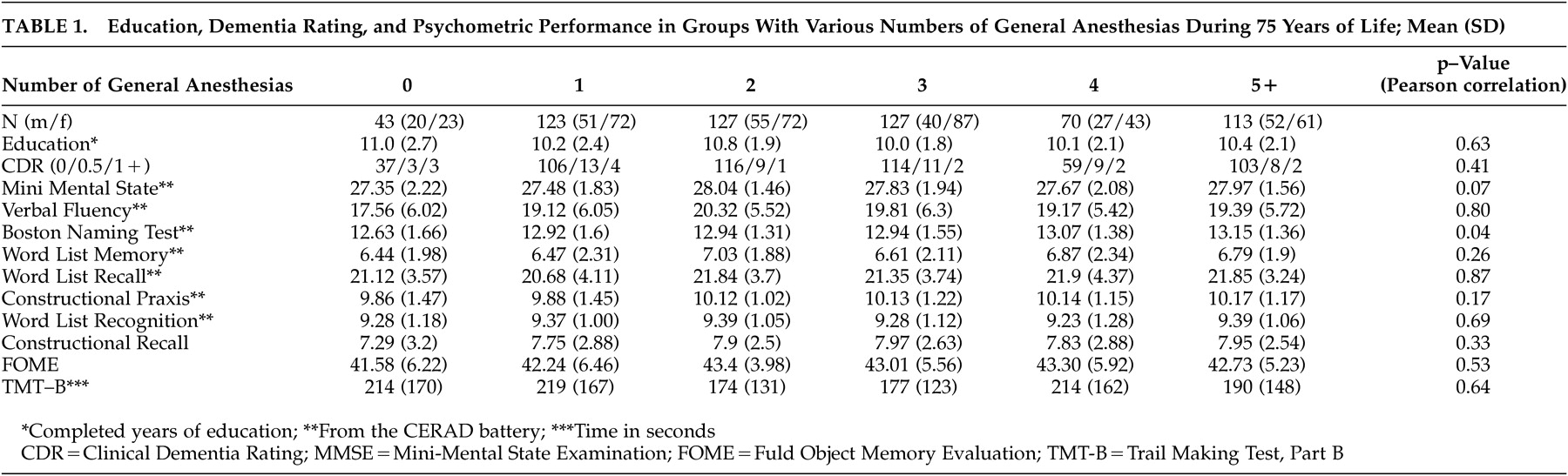
Table 1 shows psychometric scores of 10 psychometric procedures, education, and clinical dementia ratings in the groups with various numbers of general anesthesias. The mean age of this age cohort was 75.77 (SD=0.45) years.
Without correction for multiple testing, the only significant correlations show that the Boston Naming Test had lower scores associated with fewer general anesthesias. There was also a trend toward lower MMSE scores in the groups with few or no general anesthesias. Years of education were not correlated with the number of general anesthesias (r=−0.020, p=0.626; N=603) but were significantly correlated with every psychometric procedure applied except Word List Recognition (correlations not shown). Ratings on the Short Geriatric Depression Scale were not correlated with the number of general anesthesias (r=0.016, p=0.693; N=602) but were significantly correlated with every psychometric test score (correlations not shown).
Possible predictors of cognitive dysfunction used in the regression analyses showed some intercorrelations: years of education were correlated with an urban environment during childhood (r=0.28, p=0.00; N=606) and with a lower body-mass index (r=−0.15, p=0.00; N=606); mild, but significant correlations also existed between depression and education (r=–0.14, p=0,00; N=605) and between depression and atrophy of the medial temporal lobe (r=0.14, p=0,00; N=523). Male subjects had more education (r=0.29, p=0.00; N=606) and more major head traumas (r=0.09, p=0.03; N=606). All other 22 intercorrelations between predictors were far from significant.
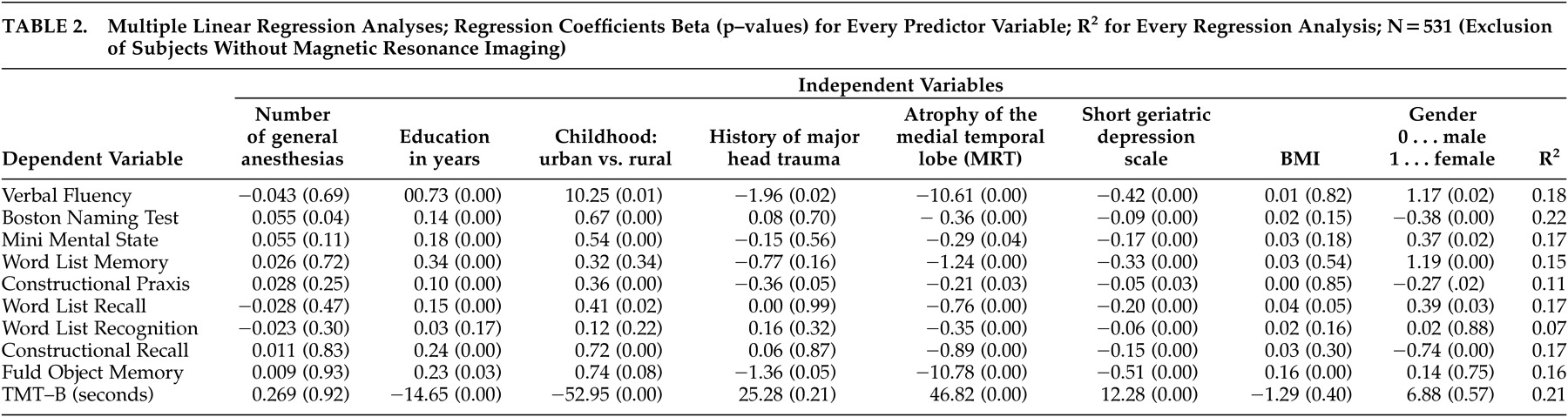
Regression analyses on test scores are shown in
Table 2 . The number of general anesthesias enters only one regression in the sense that better performance in the Boston Naming Test was predicted significantly by higher numbers of general anesthesias. No single test score was found to be lower in subjects who had a history of more general anesthesias. Lower depression self-rating and less atrophy of the medial temporal lobe predicted every psychometric score. Higher education was associated with better performance in all tests except Word List Recognition. Having lived in an urban versus rural environment during childhood predicted seven out of 10 test scores, and a positive history of major head trauma was associated significantly with three cognitive scores. Female gender predicted better performance in four verbal tests; male gender predicted better performance in two nonverbal tests and in the Boston Naming Test. Graphic expression of residuals showed a fairly symmetric distribution of residuals concerning all 10 regression analyses.
DISCUSSION
We found no indication to further promote the hypothesis that general anesthesias induce, favor, or otherwise cause cognitive deficits or Alzheimer’s disease in old age. No psychometric measure at all showed even a trend toward lower performance associated with a higher number of general anesthesias. This was the case not only for the neuropsychological procedures of the CERAD battery designed for dementia research, but also for the more difficult Fuld Object Memory Evaluation, a test of delayed verbal recall of 10 objects containing strict time limits, and for a set shifting task, the TMT-B, both of which have been described as optimally depicting postoperative cognitive dysfunction.
16Not the history of general anesthesias but other factors significantly explained psychometric test scores. We found that cognitive performance at age 75 significantly correlated with a higher level of education and higher degree of depression, as already described in the epidemiological literature.
18,
19,
22 Most cognitive performances were also associated with living in an urban environment during childhood, as described previously.
23 A positive history of major head trauma (having led to more than a 10-minute loss of consciousness) was significantly associated with lower performance on Verbal Fluency and Constructional Praxis and on the Fuld Object Memory Evaluation, as also reported in other population-based studies.
20Atrophy of the medial temporal lobe on cranial MRI was significantly associated with dysfunction in 10 out of 10 cognitive scores. Obviously, we detected some influence of preclinical Alzheimer’s disease on test performances in this community-based cohort of 75-year-olds, as medial temporal lobe atrophy has repeatedly been described as an early diagnostic marker of this degenerative dementia.
21 –
24 In comparison with all of the factors that predicted poor cognitive performance, the number of general anesthesias during a lifetime of 75 years did not at all predict impaired cognition concerning any psychometric procedure carried out.
The influence of general anesthesia on cognitive functioning in elderly people has been investigated retrospectively in a relatively fit study sample of individuals ages 24 to 86 without showing any influence of general anesthesia on age-related cognitive decline. But the influence of age on cognition and the influence of age on a number of general anesthesias made interpretations difficult.
25 Another epidemiological study assumed rather than proved that general anesthesia is a risk factor for cognitive decline.
4 A case-control study stated that general anesthesia increased the risk of developing Alzheimer’s disease by 28%.
5,
6 That general anesthesias do not increase the risk of developing Alzheimer’s disease in the VITA population will be ascertained in the entire cohort after the reinvestigation 30 months after the basic investigation took place.
The absolute number of general anesthesias was high. Ninety-three percent of subjects ages 75 and 76 had had at least one general anesthesia. One hundred thirteen subjects had undergone five or more general anesthesias. The highest number of anesthesias was 13 in two subjects, and nine subjects had undergone at least 10 general anesthesias. The 603 subjects reported a sum of 1,744 general anesthesias. We did not separate out the effects of the general anesthesias from those of surgical procedures or underlying disorders in our retrospective study because subjects with cognitive decline would be the ones with the most difficulty remembering the details. We also did not separate into anesthesias with historical drugs and modern anesthesias. But we observed a huge number of general anesthesias in a community-based age cohort, which reduces any effect of specific surgical procedures or anesthetic techniques.
We think it very probable that the type of surgery and associated complications, like blood loss and extracorporeal circulation, have an influence on postoperative cognitive dysfunction. Nevertheless, we explicitly investigated the impact of cumulative lifetime frequency of general anesthesias on cognition in a community of elderly people at age 75. Taking in mind the high cumulative exposure to general anesthesias in our population, the influence of special surgical procedures which might indeed have favored long lasting cognitive dysfunction should have been diminished.
In community-based studies of cognitive dysfunction there always remains the possibility that cognitively healthy populations are investigated due to lack of cooperation by more disabled subjects. Nevertheless, we were able to get information about current medication in a consecutive series of nonrespondents of the VITA. These subjects took insignificantly fewer medications in general and fewer medications prescribed for cognitive complaints. We think it rather unlikely that VITA participants were cognitively healthier than nonrespondents.
Education was highly correlated with every psychometric test except Word List Recognition but was not correlated with the number of anesthesias. Thus, it cannot be argued that patients with more education and general intelligence underwent more anesthesias but compensated better for cognitive dysfunction. As we applied 10 different psychometric tests and none showed any association with the number of general anesthesias, we argue that the selection of tests has not influenced these results. As described by the International Study Group on Postoperative Cognitive Dysfunction, tests of delayed recall (three tests of our battery) and set shifting (one test of our battery) are optimal to detect these cognitive dysfunctions.
16Taken together, our study strengthens the view that cumulative exposure to general anesthesia is no risk factor for cognitive dysfunction or Alzheimer’s disease in old age. Cognitive dysfunction in old age was found to be associated with low education, depression, rural environment during childhood, and a history of major head traumas. Every psychometric impairment was also associated with atrophy of medial temporal structures in MRI, indicating an influence of subclinical Alzheimer’s disease on cognition in the community-based birth cohort at age 75.
Acknowledgments
This work was supported by the Ludwig Boltzmann Institute of Aging Research, and further supported by grant P13624 from the Austrian Science Foundation FWF, Vienna, Austria.