D ementia is characterized by multiple cognitive deficits, and most patients exhibit neuropsychiatric problems while experiencing this disease.
1 Although the relationship between specific neuropsychiatric problems and the global severity of dementia is well established,
2 only a few studies have looked at relationships between specific cognitive deficits and neuropsychiatric problems. Such studies are important because insight in this relationship is relevant as the profile of neuropsychological impairments may provide information about the pathophysiological basis of neuropsychiatric problems. Moreover, a lack of awareness of the cognitive abilities or limitations of patients may adversely affect the patient-caregiver relationship, causing neuropsychiatric problems.
Several cross-sectional studies have reported associations between neuropsychological impairments and psychosis, but less is known about other neuropsychiatric problems. Studies of neuropsychological correlates of psychosis, and specifically delusions, have provided conflicting results. Though some authors found associations with frontal-temporal-related deficits,
3 –
5 others did not find more neuropsychological deficits in patients with delusions.
6,
7 Furthermore, specific associations have been found between agitation and executive dysfunction;
8 apathy and lower scores on tests of verbal memory, naming, set shifting, and verbal fluency;
9 and depression and impairments of recent memory and attention.
10 In contrast, other authors found no association between cognitive dysfunction and depression,
9 anxiety,
10 or apathy and irritability.
11Several methodological limitations, such as small samples of patients, the use of different instruments for assessing neuropsychological impairments and neuropsychiatric problems, and a cross-sectional study design, may add to these discrepancies. The aim of our study was to determine the influence of specific neuropsychological impairments on the development of a broad range of neuropsychiatric problems in patients with dementia. To our knowledge, this is the first large prospective study of this topic. We hypothesized that specific types of cognitive impairment, and in particular, language impairments, are related to more severe neuropsychiatric problems. Patients with dementia who have difficulties with expressing their thoughts and feelings may express this by means of neuropsychiatric symptoms.
METHOD
Patients
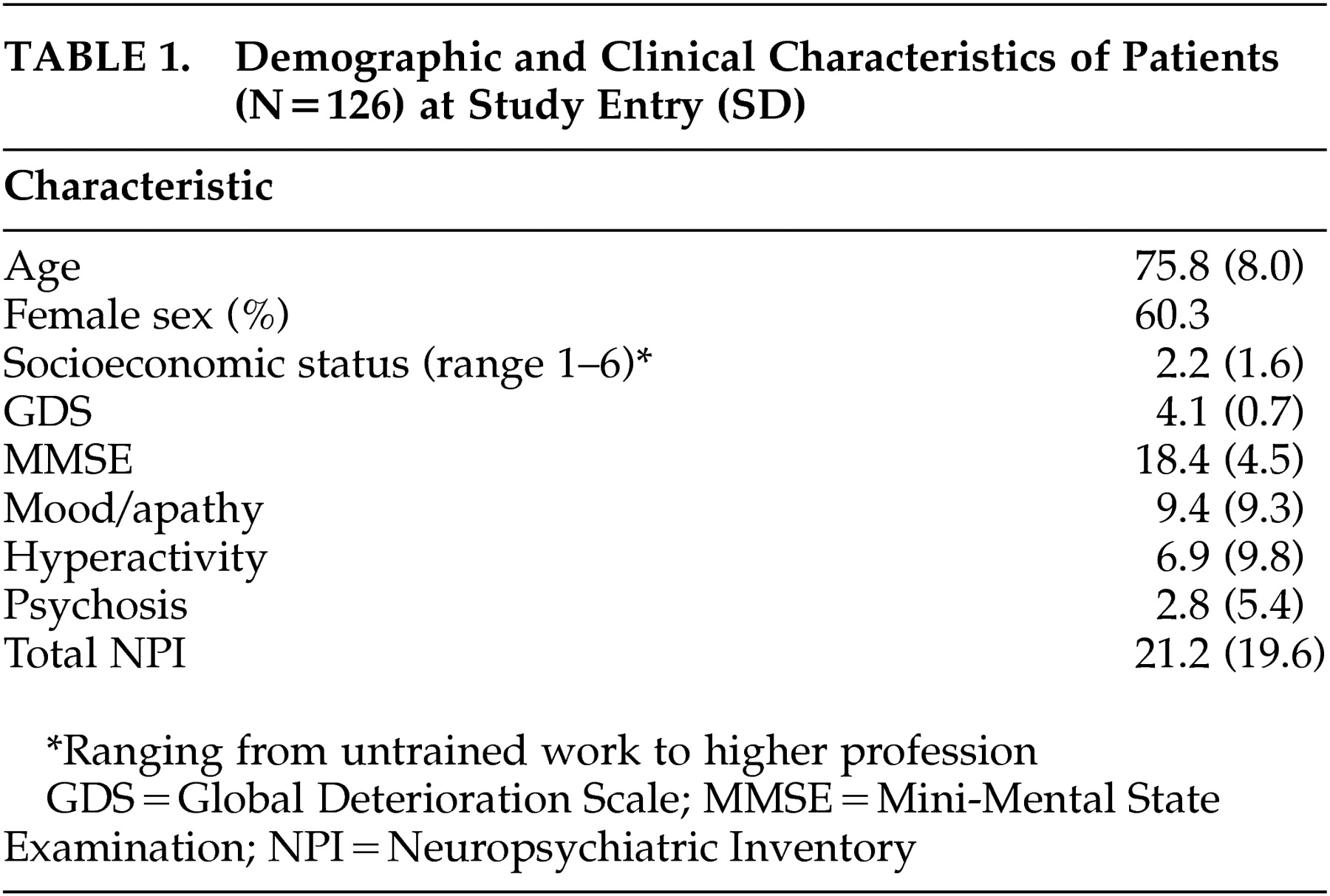
The present study was part of the Maastricht Study of Behavior in Dementia (MAASBED), a study which focuses on the course and risk factors of behavioral and psychological symptoms in dementia. MAASBED is a 2-year prospective study of 199 community-based patients with dementia who are seen at 6-month intervals. Patients were subsequently referred to the Maastricht Memory Clinic of the University Hospital or the geriatric division of the Regional Institute for Community Mental Health (RIAGG). We assessed 126 patients for whom complete 2-year follow-up data were available.
Patients were included if they met DSM-IV criteria for dementia
12 and if there was a reliable informant. One hundred patients met the NINCDS-ADRDA criteria
13 for (possible, probable) Alzheimer’s type dementia; 15 patients met the NINCDS-AIREN criteria for vascular dementia;
14 and 11 patients met criteria for dementia due to multiple etiologies. Patients were excluded if they were living in a nursing home at the start of the study. Written consent was given by the caregiver, and, when possible, by the patient. The Medical Ethics Committee of the University Hospital Maastricht approved this study.
Neuropsychiatric Problems
Neuropsychiatric problems were assessed with the Neuropsychiatric Inventory (NPI),
15 an informant-based rating scale developed to assess psychopathology in patients with dementia. The current version
16 evaluates 12 neuropsychiatric symptoms commonly observed in dementia. The severity and frequency of each symptom are scored on the basis of structured questions administered to the patient’s caregiver. The score for each symptom is obtained by multiplying severity (1 to 3) by frequency (1 to 4). The summed symptom scores give the total NPI score. The validity and reliability of the NPI
17 and its Dutch version
18 have been established.
In a previous study, principal component analysis of the NPI identified three neuropsychiatric subsyndromes: 1) a “mood/apathy” factor, including depression, apathy, night-time behavior disturbances, and eating abnormalities (four items, Cronbach’s alpha=0.63); 2) a “hyperactivity factor,” including symptoms of agitation, euphoria, irritability, disinhibition, and aberrant motor behavior (five items, Cronbach’s alpha=0.73); and 3) a “psychosis factor,” including hallucinations and delusions (two items, Cronbach’s alpha=0.72).
19 The total score of each subsyndrome was calculated by summing the NPI item scores for each factor.
Neuropsychological Assessment
In addition to general information on demographics and medical history gathered from the primary caregivers, trained research assistants administered cognitive tests to the patients.
The neuropsychological assessment covered the domains of language, memory, executive functioning, attention, orientation, perception, and praxis. Tests included:
•
A 10-item word list memory task
20 based on the Auditory Verbal Learning Test. It is an episodic memory test in which words are orally presented, one after another. Then the subject is asked to recall as many words as possible. This procedure is repeated five times, and after 20 minutes, delayed recall and recognition are tested.
•
Naming subtests of the Aachen Aphasia test
21 were used to determine impairments in naming abilities
•
The Verbal Fluency Test (categorical version)
22 measures the ability to produce as many words as possible in a fixed time span. It can be regarded as a measure for the adequate, strategy-driven retrieval of information from semantic memory. We asked the subjects to name as many animals and professions as possible within 90 seconds.
•
The Cambridge Cognitive Examination (CAMCOG), which is part of the Cambridge Examination for Mental Disorder of the Elderly (CAMDEX).
23,
24 The cognitive functions assessed with the CAMCOG included language (comprehension and expression), memory (recent, remote, and new learning), attention/calculation, praxis, abstract reasoning, perception, and orientation. It consists of 64 items with a total range of 0 (severe cognitive impairment) to 105 (no cognitive impairment). The CAMCOG is often used for the diagnosis and gradation of dementia, and one of its major advantages is the ability to detect mild forms of cognitive impairment. The Mini-Mental State Examination (MMSE)
25 score was derived from the CAMCOG.
•
The Global Deterioration Scale (GDS)
26 was used to rate the severity of dementia. The GDS is subdivided into seven stages, ranging from no cognitive deterioration at all (Stage 1) to severe cognitive and functional deterioration (Stage 7). It is one of the most widely used clinician-rated instruments to stage the course and severity of dementia. In the present study, the GDS score was included as a covariate, instead of the often-used MMSE score, because of the strong association between MMSE score and neuropsychological impairments
27 –
29 and specifically the CAMCOG.
30 In addition to cognition, the GDS covers daily life functioning and behavioral aspects. The GDS was included as a covariate to remove the effect of general cognitive decline and ensure the specificity of the findings.
Statistical Analyses
We performed statistical analyses with the Statistical Package for Social Sciences (SPSS), version 10. When a patient had only one missing assessment during the study, missing data of the NPI were imputed by either “mean substitution” of the previous and the next assessment, or “last case carried forward.” Thus, the present study involved 126 patients for whom complete 2-year follow-up data (five assessments of the NPI) were available. To determine whether cognitive performance at baseline was a predictor of neuropsychiatric problems over the 2-year period, repeated measures analyses of covariance (ANCOVAs) were performed for each of 13 different cognitive domains. These included the 10-Item Word List Task, Aachen Aphasia naming task, Verbal Fluency Test, CAMCOG total score, language (expression, comprehension), memory, attention, praxis, abstract reasoning, perception, and orientation. Patients were assigned to “low performance” and “high performance” groups according to a median-split on each cognitive neuropsychological domain as measured at baseline. “Group” (high versus low performance at baseline) was entered into the ANCOVAs as the between-subjects factor; and “time” (baseline, 6 months, 1 year, 18 months, and 2-year follow-up) was entered as the within-subject factor, after correction for global dementia severity. The following eight NPI variables were entered as dependent variables for the repeated measures analyses: the three subsyndromes, NPI total score and the separate symptoms, delusions, depression, apathy, and aberrant motor behavior. These latter symptoms were selected for separate analyses because of their high prevalence among dementia patients.
1For only 122 and 113 patients were complete data for the CAMCOG and the Word List Memory Task, respectively, available; thus, analyses with these variables had lower sample sizes. All other analyses were performed on data for 126 patients. All significance tests were performed at a two-tailed alpha level of 0.05. Because of the explorative nature of the study, Bonferroni-corrections were not made.
DISCUSSION
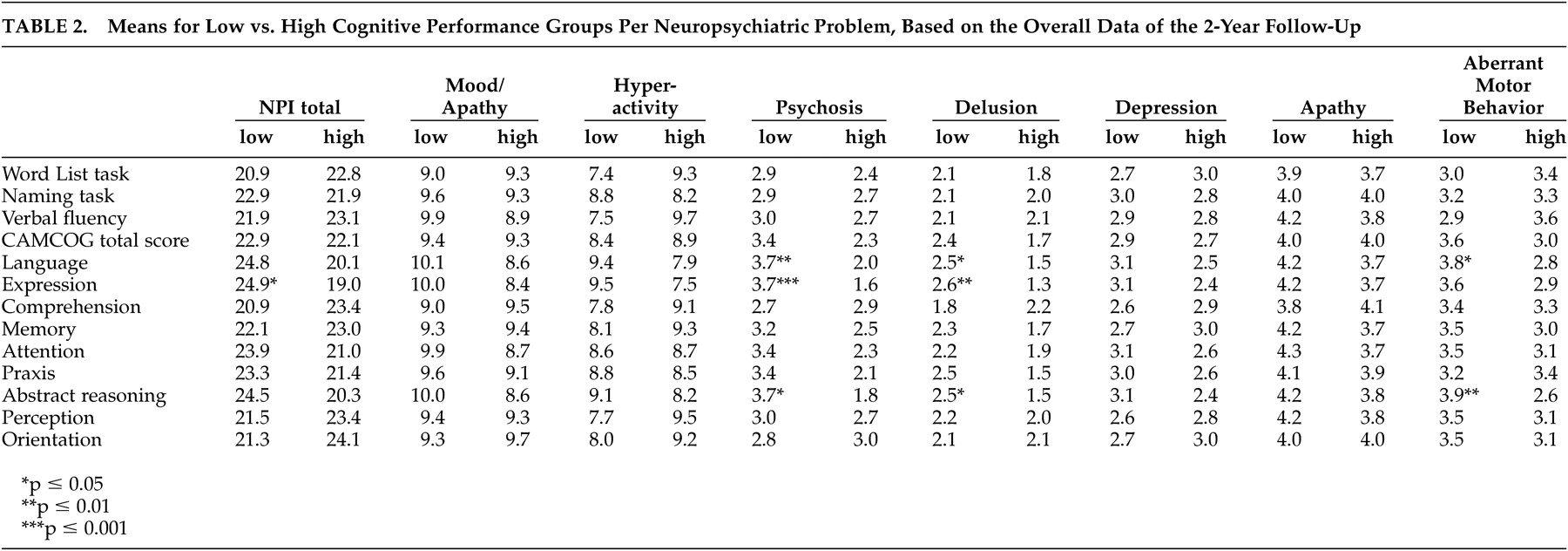
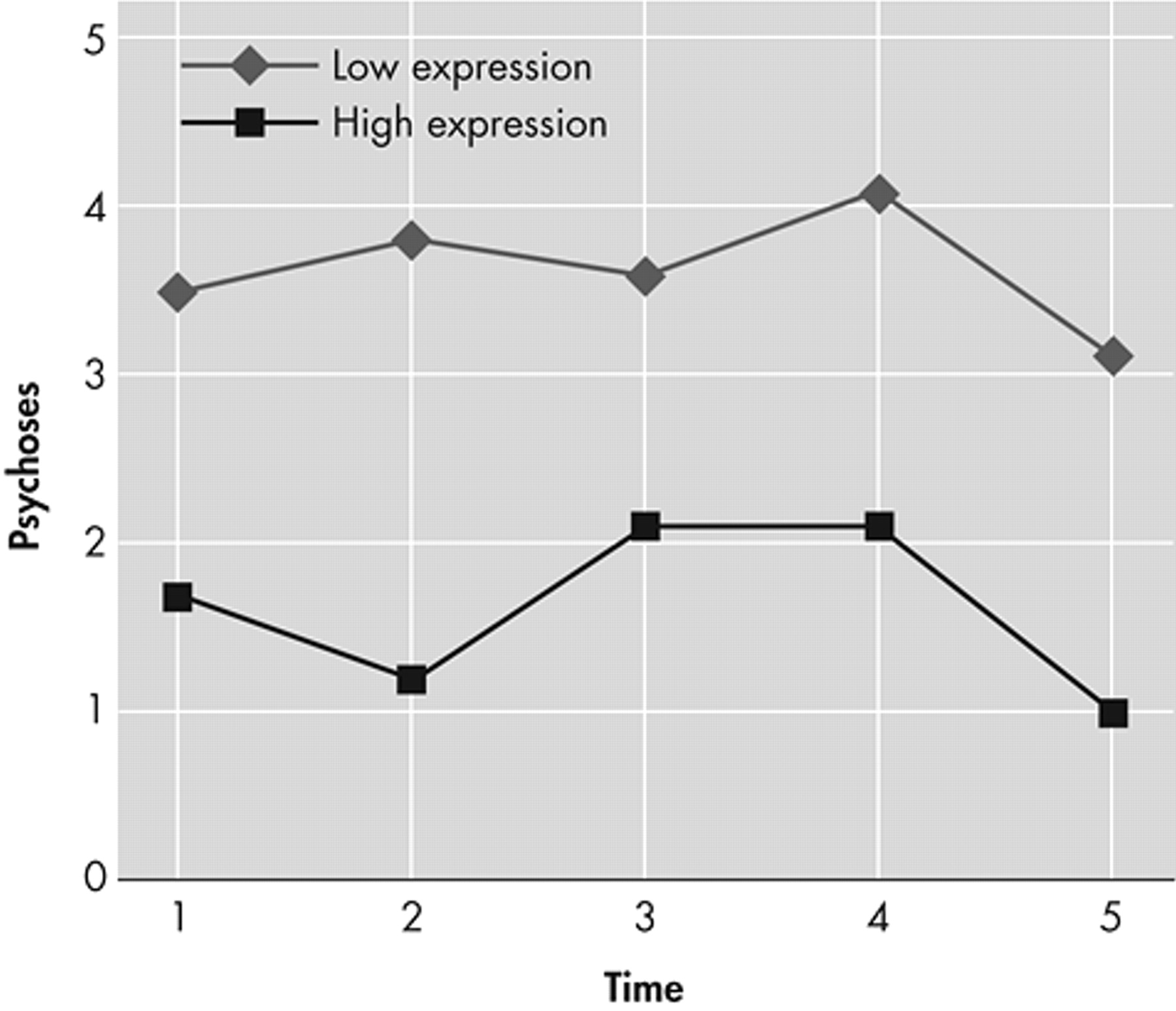
We investigated how neuropsychological functioning affected the development of neuropsychiatric problems over a 2-year period. An impairment of language expression was found to be related to overall neuropsychiatric problems, and to psychosis in particular. Aberrant motor behavior was associated with overall language impairment. These findings are consistent with those of Potkins et al.,
31 who reported an association between expressive language impairment and the presence of delusions in their cross-sectional study. In contrast to our study, they found aberrant motor behavior to be specifically associated with receptive language difficulties. We found language comprehension to be unrelated to any neuropsychiatric problem, which may be because our subjects suffered from less severe dementia than the subjects of the study of Potkins et al., which included patients living in residential homes. In addition, we measured language comprehension just with the CAMCOG, which may not have been sensitive enough for detecting comprehension impairments. Ballard et al.
32 also found an association between psychosis and expressive language, as measured with the CAMCOG. It is hypothesized that an impaired ability to communicate verbally may trigger frustration, resulting in neuropsychiatric problems, and especially psychosis.
However, further research is needed to confirm this hypothesis. As mentioned by Potkins et al., aberrant motor behavior may be the consequence of increased social isolation and diminished participation in activities as a result of poor language skills.
31 The cognitive domains for naming ability and verbal fluency had no influence on neuropsychiatric problems, suggesting that the appearance of symptoms is more related to impairments of language expression than to a dysfunction of semantic memory. The influence of verbal expression on psychosis and other neuropsychiatric problems emphasizes the role of temporal lobe lesions, with the disruption of limbic connections, in the development of these disorders. This is consistent with the results of Hirono et al.,
33,
34 who found dysfunction of temporal brain areas in Alzheimer’s patients with delusions and aggression. Language functions in the temporal lobe might involve the posterior and superior dorsal regions, while the temporo-limbic connections linked with psychosis might involve medial temporal areas.
We also found that both psychosis and aberrant motor behavior were influenced by impairments of abstract reasoning, a frontal lobe function. There is substantial evidence that patients with psychosis have frontal lobe and executive function deficits,
3 –
5,
35 and neuroimaging studies have shown dysfunction of the frontal lobes
36 –
38 and temporal areas
39 –
42 in dementia patients with psychosis. This pattern of results lends support to the theory that frontal-temporal (limbic) dysfunction may underlie the presence of psychosis, and specifically delusions, in dementia. Lopez et al.
42 suggested that dysfunction of both the frontal lobe
and the temporal lobe is needed for the development of psychotic symptoms.
Less is known about neuropsychological correlates of aberrant motor behavior. Previous research has focused on neuropsychological correlates of wandering, a symptom included in the NPI symptom “aberrant motor behavior.” A recent review of wandering behavior reported controversies about the relationship with specific neuropsychological correlates.
43 Our data suggest that neuropsychiatric problems are related to impairments of language and abstract reasoning. It remains to be determined whether aberrant motor behavior, other neuropsychiatric problems, and psychosis can be explained by a common underlying pathophysiological alteration or whether they can be explained by psychological mechanisms. Several studies have mentioned the possibility of a shared neuropathology.
6,
42,
44 Therefore, it must be kept in mind that it may be possible that the neuropsychological correlates and the neuropsychiatric problems are the consequence of the same organic defect in the brain.
Our study had several limitations. A first limitation of our study is that it was based on only 126 patients (63.3%, i.e., only 63% of the original sample had complete data). However, patients lost to follow-up did not differ in terms of severity of neuropsychiatric problems or severity of dementia at baseline from the patients who completed this study, so selective attrition seems unlikely. A second important limitation is that our conclusions must be regarded as preliminary, needing further research. Because of the explorative nature of the study, Bonferroni corrections were not made, increasing the chance of type I errors (i.e., potential for false positives) given the large number of comparisons. In addition, by dichotomizing the cognitive scores, additional information may be lost. Lastly, the lack of time effect should be mentioned. This suggests that specific forms of cognitive dysfunction at baseline did not predict the course or progression of various neuropsychiatric problems. But the main effects did suggest associations between specific cognitive deficits and neuropsychiatric problems that held constant across all time points.
In conclusion, our findings indicate that impairments of language expression and abstract reasoning are related to the emergence of psychosis, aberrant motor behavior, and total level of neuropsychiatric problems. These results imply that clinicians should be more alert to communication and executive deficits in patients with dementia, as they point to patients who are more prone to develop these types of noncognitive symptoms. In doing so, they can help patients to compensate for these problems and thereby prevent the development of neuropsychiatric problems.