T he scope and magnitude of pathological gambling have grown to become a major public health concern. Prevalence rates of the disorder range from 2.5% to 10.0%, depending on the cohort sampled.
1 –
3 Furthermore, the legal and social consequences of pathological gambling are well documented and include bankruptcy, incarceration, suicide, domestic violence and divorce, and increased risk for the onset or exacerbation of psychiatric and substance use disorders.
4,
5 While much of the research has focused on these social consequences, recent studies have sought to determine whether pathological gambling is associated with neurobiological dysfunction and whether that dysfunction is similar to the dysfunction observed in individuals with substance use disorders. For example, recent studies have shown that pathological gambling is associated with alterations in frontal lobe functioning,
6,
7 which has also been observed in individuals with methamphetamine dependence.
8,
9 Furthermore, pathological gambling and substance use disorders share similar clinical features: preoccupation, tolerance, withdrawal, and continued behavior despite negative consequences.
10 In contrast, an important distinction between substance use disorders, such as methamphetamine dependence and pathological gambling, is that impairments are present in pathological gamblers despite the lack of exogenously ingested substances. Thus, pathological gambling serves as a useful model for understanding the neurobiological alterations associated with behavioral addictions.
To date, two studies have examined the association between pathological gambling and impairment with measures that are typically used by clinicians to assess frontal lobe functioning.
11,
12 The findings of these studies are tentative, based on methodological limitations. For instance, although Rugle and Melamed
12 found that pathological gamblers performed worse than comparison subjects on untimed measures of executive function (e.g., the Wisconsin Card Sorting Test), they did not include a range of timed measures of executive function. This distinction is important with respect to differentiating whether speed of information processing mediates performance on measures of executive functioning. Moreover, Regard et al.
11 reported that pathological gamblers performed worse than comparison subjects on a range of frontal lobe measures; however, they did not statistically control for intervening variables that conceivably affected the gamblers’ test performance, such as dyslexia, developmental delays, and history of traumatic brain injury.
A subset of studies of pathological gambling has utilized a single executive measure in conjunction with a novel decision-making test in order to determine whether there was an association between performance on the two measures or whether the performances were dissociable. For example, Brand et al.
13 reported that performance on a measure of risk-taking behavior, the Iowa Gambling Task (IGT), was associated with performance on a measure of concept formation and problem solving in pathological gamblers.
14 Another study
7 using a sample of pathological gamblers and matched comparison subjects showed that reduced ventromedial cortical brain activation was associated with poorer performance on a computerized version of the Stroop test. The authors did not report whether they compared the performance of the pathological gamblers and matched comparison subjects on the Stroop test.
Other studies have addressed related questions using novel decision-making tasks and multiple comparison groups to gauge the severity of the impairments demonstrated by pathological gambling. For example, recent studies have shown that pathological gamblers perform less well than matched comparison subjects on measures of risk-taking behavior, such as the IGT.
6,
14 –
16 Petry et al.
16 further demonstrated that pathological gamblers’ performance on a delay discounting task was comparable to that of substance abusers who did not gamble. Moreover, Goudriaan et al.
15 showed that gamblers’ performance on the IGT was poorer than that of individuals diagnosed with alcohol dependence or Tourette’s syndrome.
Taken together, these studies are beginning to document the presence of neurocognitive dysfunction in pathological gambling. Still, key questions remain unanswered, such as whether pathological gambling demonstrates impairment on measures of frontal lobe functioning that are frequently used by clinicians to detect these deficits. In order to address this question, pathological gamblers in this study were administered a battery of neurocognitive tests to determine the extent of impairments on measures that are typically used by clinicians to document the presence of frontal lobe impairment. In an effort to offer a comparison of the severity of frontal lobe impairments in pathological gambling, we also assessed neurocognitive function in a separate group of individuals who met DSM-IV criteria for methamphetamine-dependence; a cohort in which frontal lobe impairment has consistently been observed
8,
17,
18 was utilized as comparison group. A third group was included and consisted of comparison subjects who were neither pathological gamblers nor methamphetamine-dependent.
METHOD
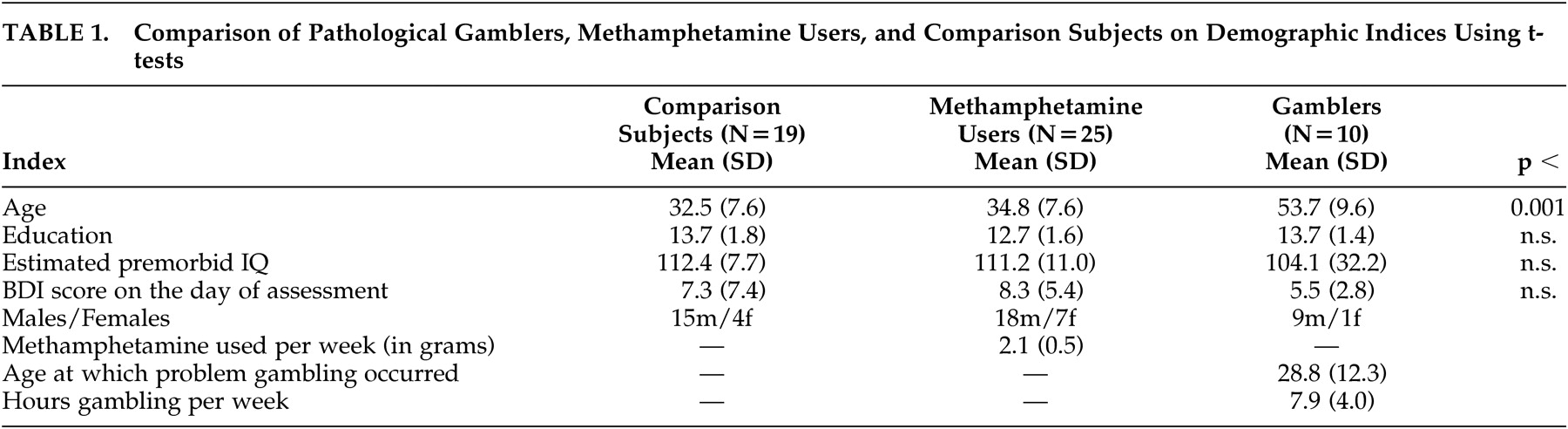
Participants included nine nontreatment-seeking pathological gamblers, 29 nontreatment-seeking methamphetamine-dependent individuals, and 19 comparison subjects. Demographics, substance use, and gambling profiles are detailed in
Table 1 . All subjects were recruited from the community through advertisements in local newspapers and flyers at local casinos. Potential participants were excluded for a history of stroke, traumatic brain injury (loss of consciousness greater than 20 minutes), epilepsy, or attention deficit disorder or for testing HIV seropositive. We used the Structured Clinical Interview for DSM-IV
10 to rule out the presence of axis I or axis II disorders. We collected urine toxicology screens from each participant to verify either the presence of methamphetamine for the methamphetamine group or the absence of illicit drugs in the comparison and pathological gambling group. Participants gave written informed consent after being apprised of the study risks and were reimbursed for participation.
The pathological gamblers met DSM-IV criteria for pathological gambling. They were required to have gambled within 2 weeks of the assessment and could not be actively involved in any formalized treatment other than Gambler’s Anonymous. Moreover, they were excluded if they met DSM-IV criteria for abuse of or dependence on any drug and/or if they had a positive urine toxicology screen.
The methamphetamine-dependent sample met DSM-IV criteria for methamphetamine dependence based on the SCID. Subjects may have used other illicit substances but did not meet DSM-IV criteria for dependence on them at the time or previously. They reported using at least 0.5 g of methamphetamine per week for the 6 months prior to the study. The route of methamphetamine administration for each of the drug users was inhalation (snorting) and smoking.
Comparison subjects did not meet DSM-IV criteria for pathological gambling. They also did not meet DSM-IV criteria for abuse of or dependence on any drug at the time or in the past and had a negative urine toxicology screen.
Once enrolled in the study, methamphetamine users were asked to discontinue use of methamphetamine. Participants were included in the study only if 1) on the date of the screening examination that was conducted within 2 weeks of the assessment, their urine tested positive for methamphetamine and tested negative for other drugs, such as cocaine, marijuana, opiates, PCP, and alcohol; 2) they produced a urine sample that was negative for methamphetamine and other drugs on the day in which the neurocognitive measures were administered; and 3) they were not experiencing clinically significant levels of withdrawal, such as insomnia, reduced appetite, or a mood disorder. The negative urine test indicated that all subjects had ceased using methamphetamine at least 5 days prior to the neurocognitive assessment, based on the elimination half-life of methamphetamine of approximately 12 hours.
19 This was considered to be a sufficient length of time for the “crash” phase, consisting of symptoms such as dysphoria, slowing, and agitation, to resolve.
Procedures
We administered to each participant a 2.5 hour battery of neurocognitive measures, which included a series of traditional frontal lobe measures. Participants were provided with breaks as needed in order to minimize testing fatigue. The screening measures included the SCID-IV,
10 the Beck Depression Inventory (BDI).
20 and the National American Adult Reading Test,
21 the latter serving as an estimate of premorbid intellectual functioning. A master’s level clinician administered the SCID. This clinician completed a 6-week standardized course regarding the SCID and was certified to administer the measure. We administered to study participants the following measures of frontal lobe functioning: Ruff Figural Fluency Test,
22 Stroop Color-Word Test,
23 and the Trail-Making Test, Part B.
24Data Analysis
We utilized multivariate analysis of variance (ANOVA) to determine group differences and to minimize the likelihood of type I error. If the multivariate ANOVA was significant, follow-up univariate ANOVAs were conducted to identify the particular measures that were most sensitive to methamphetamine dependence. Moreover, for those one-way ANOVAs that were significant, we conducted planned comparisons to determine whether the pathological gamblers performed significantly less well than the other groups. The percentage of the variance explained was calculated using eta squared.
RESULTS
Table 1 shows that pathological gamblers, methamphetamine-dependent volunteers, and healthy comparison subjects did not differ in terms of gender, education, estimated premorbid intellectual functioning, or severity of self-reported depressive symptomatology. Although the groups differed with respect to age, covariate analyses revealed that age did not correlate with performance on the neurocognitive measures; thus, multivariate ANOVA was utilized to measure group differences in test performance. The multivariate ANOVA revealed that the gamblers, methamphetamine dependent volunteers, and nondrug-using comparison subjects differed significantly with respect to their performance on the frontal lobe measures (F=3.95, df=6,98; p≤0.01). Eta squared, which estimated the magnitude of the group differences, was 0.20, which is considered to be large.
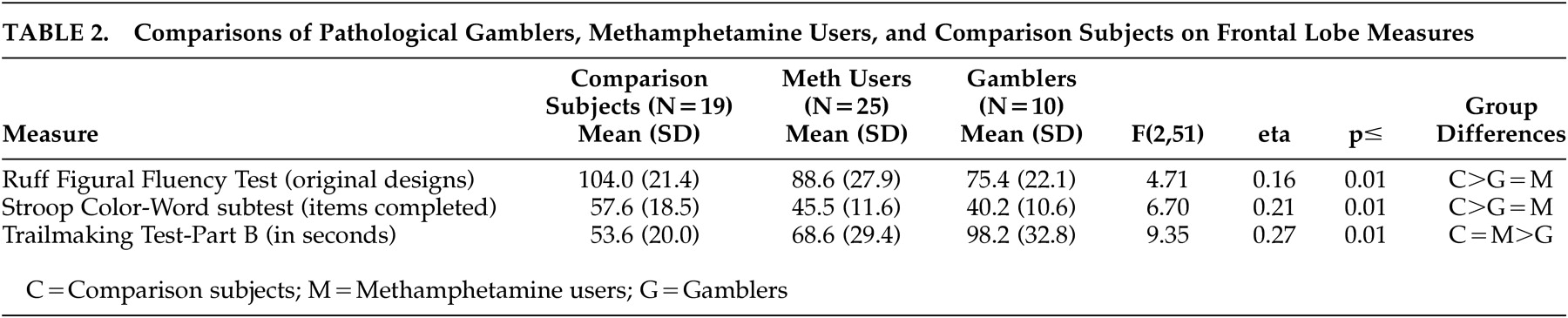
Table 2 shows that the groups differed significantly on each of the three frontal lobe measures. The pathological gamblers performed significantly less well than the comparison subjects on each of the three measures (p<0.05). The performance of the pathological gamblers was similar to that of the methamphetamine users on the Ruff Figural Fluency Test and the Stroop Color Word Test but significantly worse than the methamphetamine users on Trail-Making Test, Part B (p<0.01). Eta squared, which estimated the magnitude of the group differences, ranged from 0.16 to 0.27, which is considered to be moderate to large.
DISCUSSION
These findings are the first, to our knowledge, to demonstrate that pathological gambling is associated with impairments across a range of timed measures that are frequently used by neuropsychologists for the purpose of documenting frontal lobe dysfunction. This finding implicates abnormalities in frontal-subcortical pathways, which mediate performance on these measures.
25 Future studies, such as those using simple and complex measures of reaction time, will assist in determining whether poor performance on these measures is the result of slowed information processing and/or a frontal lobe defect, per se. For example, slowed information processing may interfere with the ability of pathological gamblers to process all of the information presented to them; as a result, they will be at risk to make decisions without considering information that potentially is salient to those decisions.
Based on the findings from related studies, we can speculate regarding the specific mechanism(s) of the frontal impairments in pathological gambling. For example, neuroimaging studies have shown that pathological gamblers demonstrated decreased blood oxygenation levels in left ventromedial cortex using fMRI.
7 Moreover, Reuter et al.
26 replicated and expanded upon this finding by showing that hypoactivation of ventral striatal and ventromedial prefrontal regions was associated with disease severity.
Though these findings preliminarily show that pathological gambling is associated with frontal impairment, several limitations should be noted with respect to the current study. For example, the pathological gamblers were older than the volunteers in other groups. Although this issue was addressed using covariate analysis and by determining that age was not associated with test performance in this sample, it would be helpful to include an age-matched sample in future studies. Additionally, the sample size was not large enough to identify risk or protective factors distinguishing between impaired and nonimpaired gamblers. Likely risk factors for frontal lobe impairment that should be considered in future studies might include frequency or duration of pathological gambling, or demographic factors, such as age, education, and socioeconomic status, and other comorbid disorders, such as attention deficit hyperactivity disorder, which affects a disproportionate number of pathological gamblers relative to the general population.
27 –
29 In addition, it may be useful to conduct collateral interviews and/or obtain collateral data sources (e.g., previous treatment records) in order to verify the self-reports of study participants. Moreover, the sample size limits the degree to which these findings may be generalized to gamblers with different demographic profiles, such as younger or older pathological gamblers with higher levels of education. Furthermore, serial assessments in abstinent individuals with pathological gambling will clarify the durability of these findings.
At present, the etiology of the frontal lobe deficits remains unclear. Because the study design was cross-sectional, it was not feasible to address this issue in a conclusive manner; however, by recruiting gamblers with limited comorbid psychopathology, these findings tentatively suggest that pathological gambling is a risk factor per se for frontal lobe dysfunction. Serial assessments of gamblers without comorbid disorders who are not abstinent or who are abstinent for various lengths of time will assist in providing additional insight into the manner in which pathological gambling affects frontal lobe function. Moreover, serial assessments might also clarify the particular association between potential changes in frontal lobe function and changes in gambling strategies. For example, if serial assessments reveal deterioration in frontal lobe functioning, it will be interesting to determine whether the gamblers are more likely to utilize poor strategies and/or behave more impulsively.
Findings from this study carry several implications for future work. First, future studies need to address how these neurocognitive impairments relate to pathological gambling behaviors. It is possible that these deficits are responsible for persistent gambling through a failure to inhibit behaviors or perhaps through an inability to adapt to continual losing. In addition, future studies need to determine how these measures of frontal lobe functioning can assist clinicians with regard to providing focused care for their patients. Additionally, if the impairments persist following the cessation of pathological gambling, then future studies might consider the use of reconceptualizing psychotherapeutic interventions as a means of circumventing them
30 –
32 as well as identifying medications that can ameliorate them.
Acknowledgments
This study was supported by grants from the National Institute on Drug Abuse (K24 DA17754, R25DA014593, R03DA020591) and a gift from the Annenberg Foundation.