D ivalproex sodium and its metabolite, valproic acid, are anticonvulsant drugs used in the treatment of mania and seizure disorders.
1,
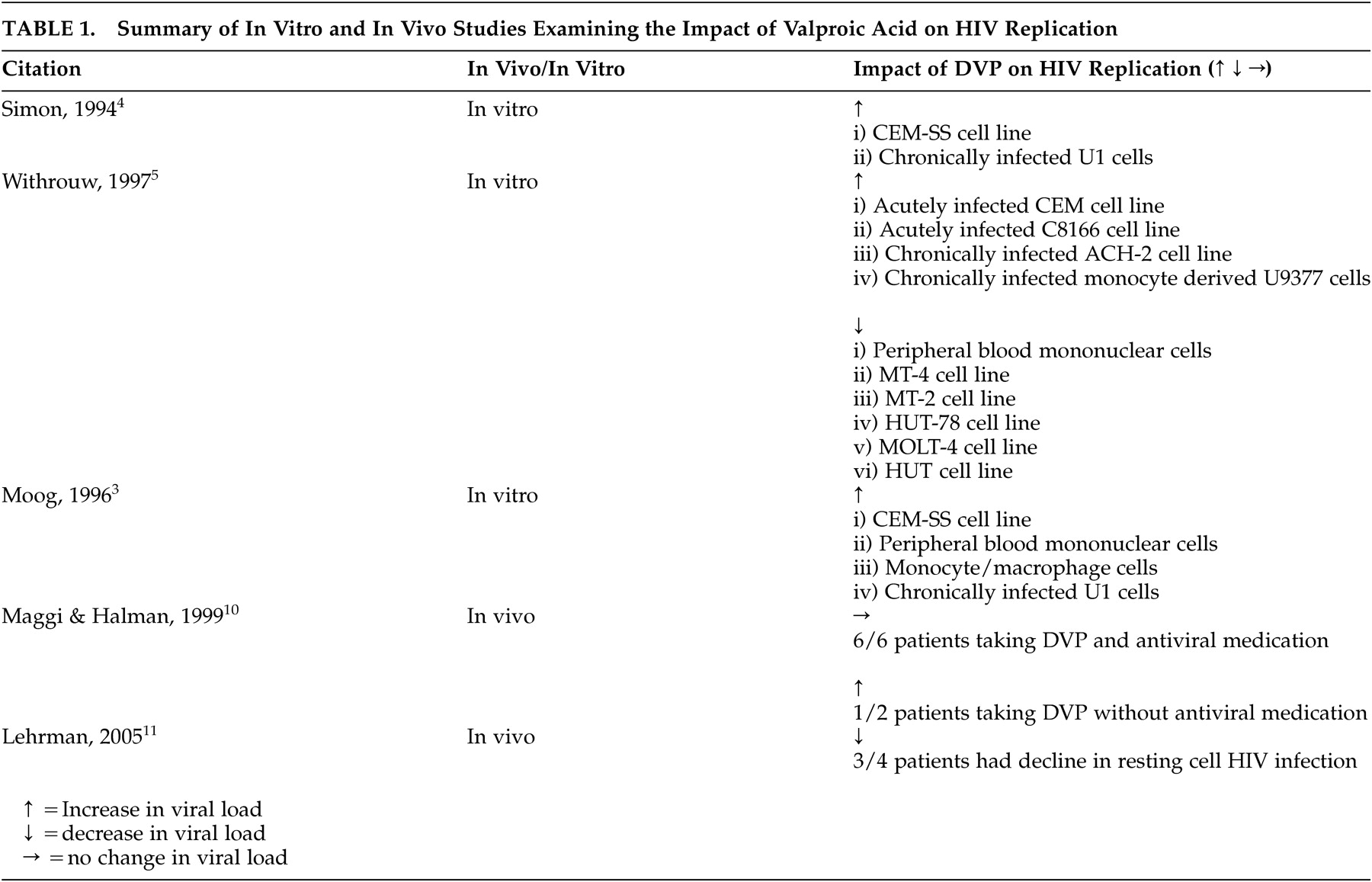
2 Initial in vitro data showed that valproic acid activates HIV replication at concentrations frequently reached in the plasma of valproic acid-treated patients.
3 –
5 One postulated mechanism for this increase in replication is through the inhibition of glutathione reductase, resulting in lower levels of glutathione.
4 Study results have been inconsistent, with valproic acid activating HIV replication in some cell lines but not in others.
3 –
5 Nonetheless, the findings raised concerns about the use of valproic acid in HIV-positive patients,
6 –
9 in particular, the potential for an increase in HIV viral load and the potential emergence of viral resistance.
8 Clinical evidence addressing this issue is limited. One retrospective study
10 examining 11 patients found that divalproex sodium administration in the presence of antiretroviral treatment did not cause a clinically meaningful increase in HIV replication. This study was limited by its small sample size and its retrospective nature, which resulted in incomplete follow-up data on other clinical factors that may have affected HIV viral load. More recently, an in vivo study by Lehrman et al.
11 postulated a potentially beneficial impact of valproic acid on HIV replication, noting the ability of valproic acid to deplete HIV infection in resting CD4+ T-lymphocyte cells in vivo, which may lead to improved viral suppression when valproic acid is used in conjunction with antiretroviral medication. This small proof-of-concept study showed that valproic acid induced the expression of HIV from the resting CD4+ T-lymphocyte cells of aviremic patients but did not activate new infection.
11 Table 1 summarizes the results of the in vitro and in vivo studies.
In order to further understand the clinical impact of the use of divalproex sodium in patients with HIV disease, we undertook this study to prospectively determine whether divalproex sodium causes an increase in HIV replication over a 1-month period when administered to patients with HIV disease for treatment of mania/bipolar disorder, or dementia with behavioral distrubances secondary to HIV.
Our hypothesis, based on the retrospective review
10 as well as our clinical experience, was that divalproex sodium would not increase HIV replication sufficiently to produce a clinically significant increase in HIV viral load, particularly if the patient were receiving highly active antiretroviral therapy (HAART).
METHOD
Our study used an observational prospective design. We asked all patients 18 years of age and older who had HIV disease and presented to the HIV Psychiatry Program of St. Michael’s Hospital to participate in our inception cohort, provided they met the following criteria:
1.
The patient had a current clinical diagnosis of a manic/hypomanic episode, bipolar mood disorder, dementia with mania or behavioral disturbances secondary to HIV infection.
2.
The patient made a decision with his or her treating psychiatrist to begin treatment with divalproex sodium.
In keeping with the observational nature of our study, all treatment decisions were made between the treating psychiatrist and the patient. Divalproex sodium dosing was flexible with the titration schedule created at the discretion of the treating psychiatrist, aiming for a usual daily treatment dose of 1000 to 1500 mg/day. Final stable treatment dose was established based on symptom control, side effect profile, and valproic acid level.
Study personnel assessed each participant prior to initiating treatment with divalproex sodium (visit 1), at day 5 after divalproex sodium therapy was initiated (visit 2), and at day 5 after a stable treatment dose of divalproex sodium was achieved (visit 3). The time elapsed between visits 1 and 3 ranged from 2.1 to 5.1 weeks with a mean of 3.2 weeks.
Stable treatment dose was defined as the optimal dose to control symptoms in an individual patient. The measurement intervals were chosen to reflect time-to-steady-state divalproex sodium drug levels, and to closely approximate intervals used in the in vitro studies, which showed that valproic acid increases HIV replication 5 to 14 days after exposure to divalproex sodium.
3 –
5 In addition, the measurement intervals reflect the Guidelines for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents,
12 which suggest that viral load be tested at 2 to 8 weeks after initiating or changing antiretroviral therapy, as a decision point for monitoring the impact of changes to a therapy regimen.
In our study, an increase in Log
10 HIV viral load ≥0.5 was used as a marker for a clinically significant increase in HIV viral load. This is consistent with the current
Guidelines for the Treatment of HIV Disease in Adults and Adolescents, 12 which uses this value to define virological failure and clinical progression.
Participants completed a demographic/clinical questionnaire at visit 1. At all visits, participants completed mood rating scales, including the Clinician Administered Rating Scale for Mania
13 and the Beck Depression Inventory,
14 and a 4-day retrospective, medication adherence self-report questionnaire (adapted from the University of California at San Francisco’s Adherence Follow-up Questionnaire for HIV Disease);
15 we measured their HIV viral load using the Quantiplex HIV RNA 3.0 Assay (Chiron Corporation).
16 Valproic acid level testing was completed during visits 2 and 3.
Participants were paid $10.00 per visit as remuneration for participation, and up to an additional $10.00 per visit to cover travel costs. Our study was approved by the Research Ethics Board of St. Michael’s Hospital. Voluntary informed consent was obtained from each participant.
RESULTS
Eight patients were enrolled in our study, including five with a manic or hypomanic episode, two with bipolar mood disorders who were making changes to their mood stabilizers, and one with dementia secondary to HIV infection, with behavioral disturbances.
We evaluated the eight study patients between May 1999 and January 2001. They were primarily male (7:1) and were an average age of 43.50 (SD=10.18) years. Their average baseline CD4+ cell count was 156.88 (SD=187.97) cells/mL, and their average baseline viral load was 125,468.75 (SD=173,815.96) copies/mL. One patient had a nondetectable viral load at baseline (i.e., viral load<50 cells/mm 3 ). HIV disease staging using the 1993 Centers for Disease Control classification system was A (asymptomatic) N=2, B (symptomatic, non-AIDS) N=2, and C (AIDS) N=4. The average time since their HIV diagnosis was 10.88 years (SD=2.85 years). Four patients were taking antiretroviral therapy at visit 1, and a fifth patient began antiretroviral therapy between visits 2 and 3.
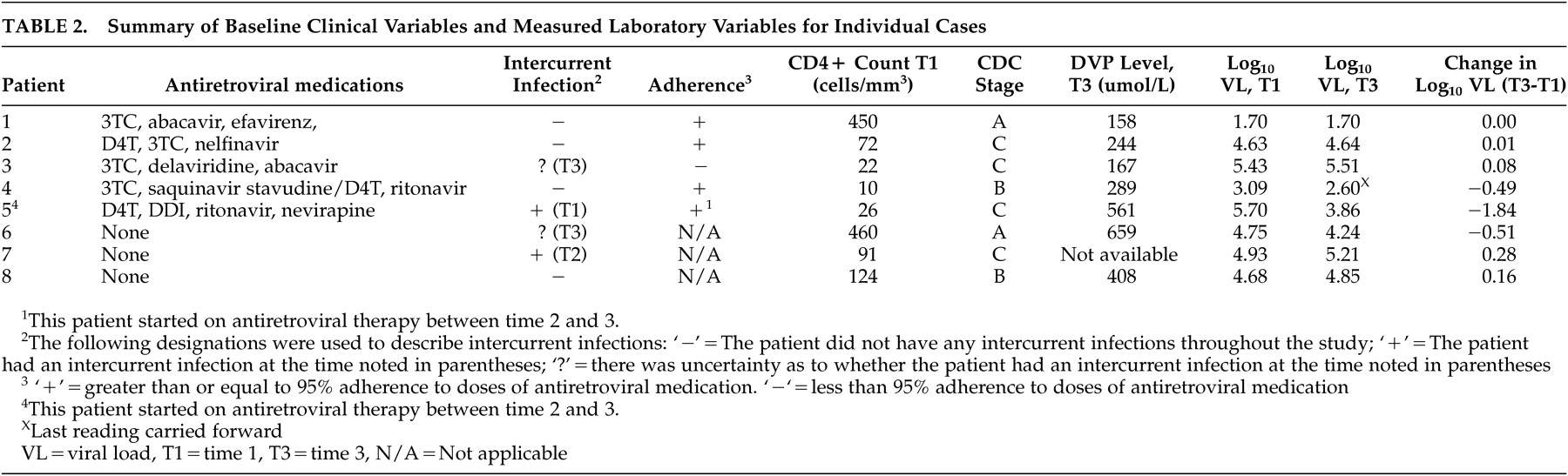
The summary of baseline clinical and measured laboratory variables for individual cases is listed in
Table 2 .
One patient had a nondetectable viral load, and it remained nondetectable over the course of the study. Three patients had a decrease in viral load, and four had an increase in viral load. The decrease in viral load for patient 5 coincided with the start of antiretroviral therapy. Of the patients with an increase in viral load, those taking antiretrovirals had the smallest increases. Their Log 10 HIV viral load increases did not exceed 0.08. Their average change in Log 10 HIV viral load was −0.29 (SD=0.69). The proportion of participants with an increase in Log 10 viral load ≥0.5 was 0/8 (95% confidence interval=0% to 34%). No patients stopped divalproex sodium use due to medication intolerance.
DISCUSSION
This study prospectively examined the clinical significance of in vitro data showing that HIV replication increased with exposure to valproic acid.
3 –
5 In this study, no patient had an increase of Log
10 viral load ≥0.5. Four individuals in this series had a smaller increase in HIV viral load. The impact of this small increase on clinical HIV disease progression is unclear. No study patients who were without antiretroviral medications at visit 1 had an increase in viral load sufficient to necessitate starting antiretroviral therapy, and no patients taking HAART at visit 1 had an increase in viral load sufficient to necessitate a change in their antiretroviral regimen. The largest increase in HIV viral load (0.28 and 0.16 Log
10 viral load) occurred in two individuals who were not taking antiretrovirals. A third individual, whose antiretrovirals were stopped for 12 days prior to visit 3, had the next highest increase in viral load, at 0.08 Log
10 viral load. It is possible that the role of antiretroviral medication, and strict adherence to that regimen, is important in limiting the impact of any divalproex sodium drug-virus interaction.
The pilot nature of this study does not allow us to make firm conclusions from these data; however, we postulate that the concerns raised by the in vitro studies demonstrating up-regulation of HIV replication may not be clinically significant. Other factors, including the body’s natural immune response and the use of antiretroviral medications, likely offset any significant interaction. Alternatively, the 2005 suggestion by Lehrman et al.
11 of the benefit of valproic acid, when used with antiretrovirals, in inducing expression of HIV from resting CD4+ T-lymphocyte cells may be the more important drug-virus interaction in the in vivo situation.
Our results must be interpreted and applied with caution, given the limitations of our study design, small sample size, short length of follow-up, clinical heterogeneity of sample, and absence of a comparison group. It is possible that a true increase in viral load may have occurred as a result of the drug-virus interaction, which we failed to detect due to our small sample size, low power, and short follow-up duration. Enrollment in this study was lower than anticipated and may have been due to a decreased prevalence of late-stage HIV disease complications seen in the era of widespread availability of antiretroviral medications, including lower rates of HIV mania and HIV dementia. As primary and secondary mania remain a significant psychiatric complication in HIV-positive patients in resource limited countries,
17 it is important that future research be undertaken to more definitively address this question using a larger patient sample enrolled in a multisite double-blind, randomized, control trial.
Acknowledgments
This research was supported by an operating grant from the Ontario HIV Treatment Network. This paper was presented as an abstract at the Academy of Psychosomatic Medicine 48th Annual Meeting in San Antonio, TX, November 2001, and received an award for “Best poster by a trainee.”