P oststroke depression has become a focus of research in many countries of the world.
1 –
3 Studies utilizing either psychological
4 or pharmacological treatments
5 –
8 for poststroke depression have been published during the last two decades. At the present time, there are eight randomized, double-blind, placebo-controlled treatment trials, two double-blind, nonplacebo-controlled studies, and 13 open label studies of poststroke depression.
9 In addition to these pharmacological studies, two retrospective studies have examined the utility of ECT
10,
11 and one study
12 has examined the utility of transcranial magnetic stimulation in the treatment of poststroke depression. The study duration was 12 weeks in only two of the double-blind trials, while the remainder had study durations ranging from 3 to 6 weeks.
9 In spite of the fact that nortriptyline, citalopram and sertraline have all been demonstrated to produce significant improvement in poststroke depression, the Federal Drug Administration (FDA) has not approved a drug with an indication for this condition. In addition, all of the medications which have been used in the treatment of poststroke depression have been used in the treatment of depressive disorders without stroke. With the exception of Kimura et al.
13 who treated 12 poststroke depression patients with milnacipran in a 6-week trial, and Hougaku et al.
14 who treated 20 poststroke depression patients using lisuride in a 12-week trial, all of the study drugs have been tricyclics, selective serotonin reuptake inhibitors (SSRIs) or methylphenidate. Another new compound, however, which has been studied for treatment of depression following stroke is nefiracetam. Nefiracetam is a novel cyclic gamma aminobutyric acid (GABA) compound which has been demonstrated in animal studies to enhance aminergic, glutamatergic, and cholinergic neurotransmission by stimulating alpha 4 beta 2 type neuronal nicotinic acetylcholine receptors.
15 –
20 In addition, nefiracetam increased brain-derived neurotrophic factor expression as well as regional blood flow and glucose utilization after sustained cerebral ischemia in rats.
21,
22 This compound was first administered to humans in Japan as a potential treatment for poststroke sequelae, such as cognitive impairment, through increased brain blood flow. Preliminary results published in Japanese-language journals, however, showed a treatment effect on outlook and interest but no significant change in cognitive impairment.
23The current study was therefore undertaken as part of a phase II trial of nefiracetam, primarily for treatment of poststroke depression and secondarily for apathy utilizing multisite enrollment and double-blind, placebo-controlled methodology. We tested the hypothesis that 900 mg of nefiracetam would have the greatest benefit on depression, while 600 mg would represent the lowest effective dose to treat depression compared with placebo.
METHOD
Study Patients
This multicenter trial (28 sites) was conducted in the United States and Canada between 1999 and 2001. A total of 159 patients with a DSM-IV diagnosis of “depression due to stroke with major depressive-like episode” and Hamilton Depression Rating Scale (HAM-D) score of ≥18 were enrolled between 10 days and 3 months following acute stroke. Exclusion criteria were subarachnoid hemorrhage, other psychiatric or neurological disease such as Alzheimer’s disease or Parkinson’s disease, depression or suicidal plans which require psychiatric hospitalization, existence of other life-threatening conditions, comprehension deficit that would preclude a verbal interview, and taking any other psychotropic medication with the exception of benzodiazepines or other related insomnia medications. The protocol and possible side effects of nefiracetam were fully explained to eligible subjects and those who provided informed consent were enrolled in the study. Institutional review board approval was obtained at each enrollment site. Patients were randomly assigned to one of three treatment arms: 600 mg nefiracetam (N=55), 900 mg nefiracetam (N=48), or placebo (N=56). This was the intent-to-treat group. Nefiracetam or placebo was administered double-blind in three identical 150 mg capsules twice per day. This was necessary because the half-life of nefiracetam is approximately 6 hours. Patients were evaluated prior to entry into the study and followed up at 4 weeks, 9 weeks, and 12 weeks, at which time the study was completed and the nefiracetam was discontinued.
Diagnosis
All patients were required to meet diagnostic criteria for depression due to stroke with major depressive-like episode based on evaluation using the Present State Examination,
24 a semistructured mental status examination which was modified to elicit reliable DSM-IV diagnoses.
Psychopathological Evaluation
In addition to the Present State Examination, all patients were assessed with the HAM-D (17 item),
25 which has been demonstrated to be reliable and valid as a measurement of severity of poststroke depression.
26 This scale was intended to be the primary outcome measure. In addition, the Beck Depression Inventory (BDI)
27 was administered as a self-rated depression measure. This has been demonstrated in previous studies to be a reliable and valid measure of depression in the stroke population.
28,
29Prior to beginning this study and halfway through the study, all study raters were trained in the use of these instruments. All raters were shown five videotapes of poststroke depression patient interviews utilizing these instruments. They were asked to rate the patients’ responses. The interview rater was not permitted to participate in this study until his or her interrater agreement exceeded 80% when compared to the videotape ratings of an experienced interviewer (i.e., RGR).
Neurological and Cognitive Evaluations
Patients were administered the Modified Mini-Mental State (3MS).
30 This is a 100-point scale with lower scores indicating greater cognitive impairment. It has been used to assess patients with stroke and has been demonstrated to be both a valid and reliable assessment of cognitive function.
31 The Functional Independence Measure
32 has been used to assess impairment in activities of daily living and is widely used in rehabilitation research. It is a scale which has been shown to be reliable and valid in the assessment of functional impairment in patients with stroke.
32 The NIH Stroke Scale
33 was used to quantify the severity of neurological impairment following stroke. This instrument has been widely used in studies of patients with stroke and provides a quantitative assessment of the severity of motor, sensory, and language impairment associated with stroke.
33 These measures of cognitive, physical, and language function were used to ensure comparability of severity of stroke across the treatment groups and because prior studies have shown that treatment of poststroke depression can improve physical and cognitive function.
34,
35Statistical Analysis
Primary analysis was done in the intent-to-treat group (N=159) and secondary analysis was done in the group designated to evaluate efficacy, who took nefiracetam for at least 4 weeks (N=139). Data analysis was conducted using means, standard deviations, and analysis of variance (ANOVA). Longitudinal data were analyzed using repeated measures ANOVA.
Intention to treat analysis utilized the last observation carried forward approach. Nonparametric analysis was carried out using chi-squared or Fisher’s exact. Statistical significance was based on a two-tailed p value of less than 0.05. Although this study included comparisons of 10 measures across three groups, there were only two essential comparisons: repeated measures ANOVA of time by treatment interaction of HAM-D scores and BDI scores. Thus, we did not adjust the level of significance.
RESULTS
A total of 159 patients were randomized. There was one patient who dropped out before receiving any medication. Within the first 4 weeks of treatment, five placebo and two treated patients (900 mg) dropped out. Because of failure to take their medications regularly or protocol violations (e.g., some developed physical illness), one placebo, eight 600 mg treated patients and five 900 mg treated patients were withdrawn prior to the first posttreatment evaluation at 4 weeks. Thus, 137 patients had at least 4 weeks of treatment and were included in our efficacy analysis.
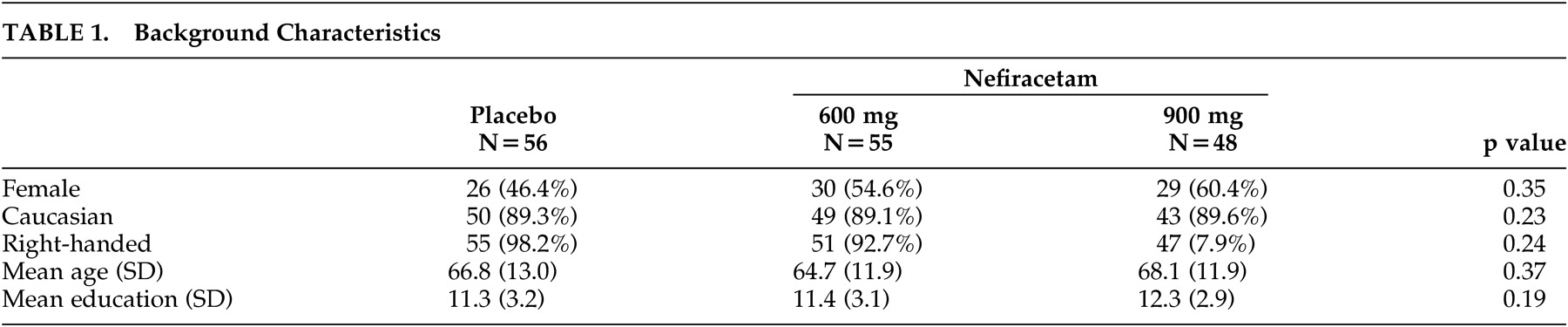
Background Characteristics
The background characteristics of the 159 patients in three treatment groups are summarized in
Table 1 . There were no statistically significant differences in any of the background characteristics. The average patient included in the study was male, was in his mid-60s, and had an 11th-grade education.
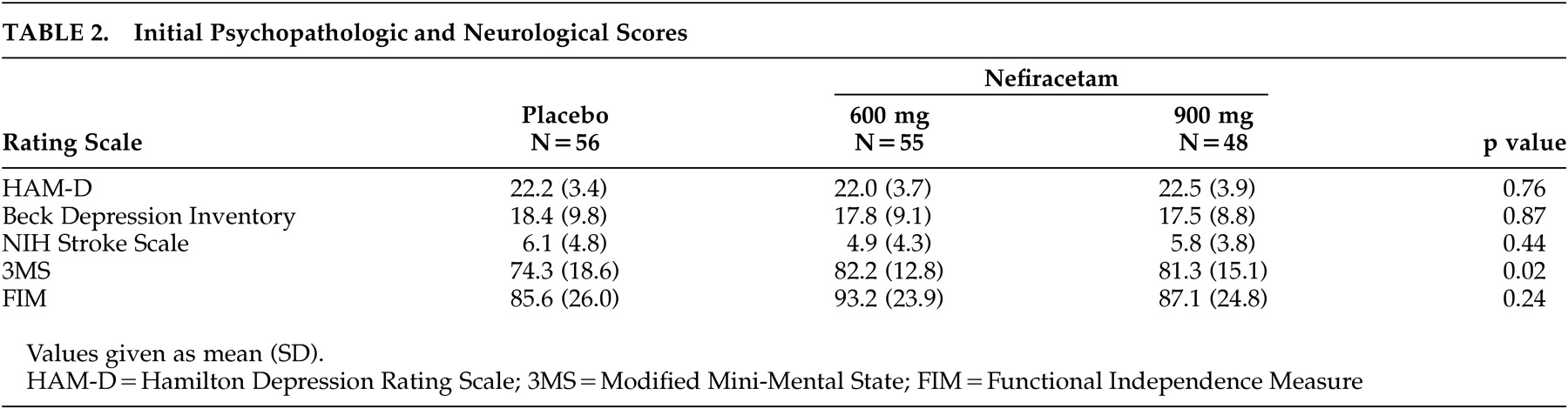
Psychopathological and Neurological Findings
The mean and standard deviation scores for each of the scales administered divided by treatment groups are shown on
Table 2 . There were no significant differences among the three treatment groups in their initial scores on the HAM-D, the BDI, the Functional Independence Measure, or the NIH Stroke Scale. The patients who received placebo, however, were significantly more impaired prior to treatment than the patients receiving nefiracetam in their cognitive function as measured by the Modified Mini-Mental State test.
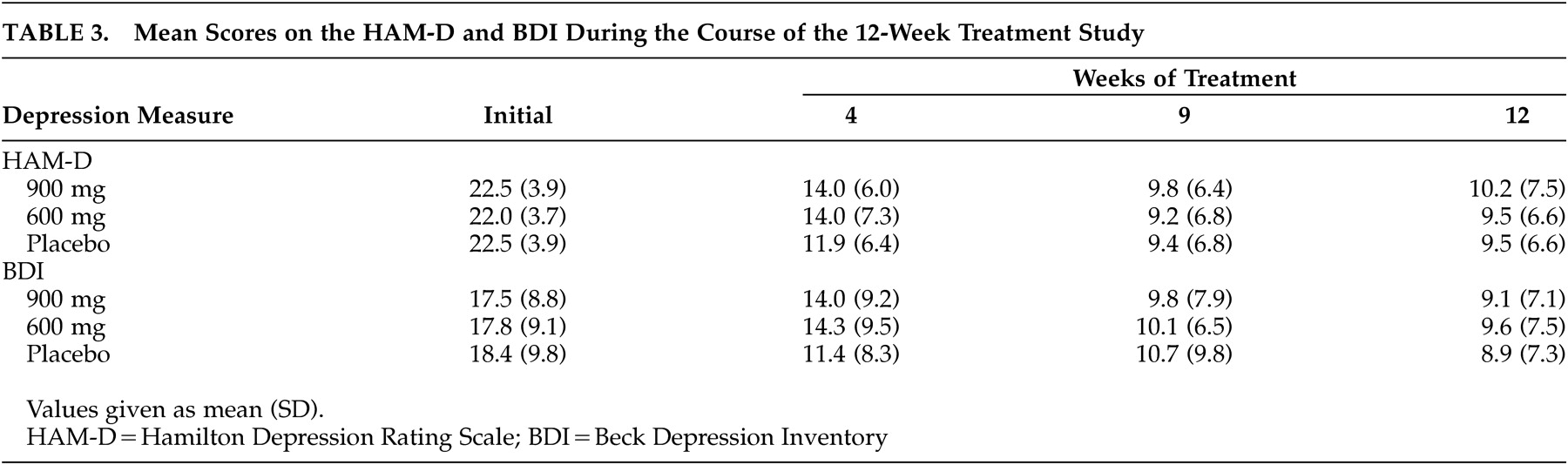
Effect of Treatment
The HAM-D scores during the course of the 12-week treatment study using intention to treat analysis are shown in
Table 3 . There was a significant effect of time; however, the time by treatment interaction failed to reach statistical significance (F=0.81, df=6, 308, p=0.56). Similarly, based on efficacy analysis (i.e., 4-week or more evaluations), there was no significant time by treatment effect of 600 mg (N=46) or 900 mg (N=44) of nefiracetam compared with placebo (N=50) (F=0.52, df=6, 236, p=0.79). We also conducted an item by item analysis of the HAM-D and found no significant treatment effects. We examined for site effects among the three sites that enrolled more than six patients and found no differences in response rates between these sites and the overall response rates. Furthermore, using efficacy analysis, there was no significant time by treatment interaction on the BDI (F=1.35, df=6, 236, p=0.25), the Functional Independence Measure (F=1.23, df=6, 226, p=0.29), the Modified Mini-Mental State (F=1.74, df=6, 204, p=0.11) or the NIH Stroke Scale (F=0.38, df=6, 216, p=0.82).
Based on the fact that effect size of active versus placebo treatment has been found to be higher among patients with more severe depression,
36,
37 we analyzed treatment response among the most severely depressed quintile of patients (N=9, 600 mg; N=7, 900 mg; N=12, placebo) using HAM-D scores. The selection of top quintile was arbitrary but is frequently used by investigators. All pretreatment HAM-D scores were 25 or greater. There was a significant time by treatment interaction (F=3.24, df=6, 46, p=0.010). Individual post hoc group comparisons showed that the 900 mg group had significantly lower HAM-D scores at 12 weeks of treatment (t=1.7, df=17, p=0.05, one-tailed) compared with the placebo group. There were no significant differences between 600 mg and placebo groups. The 900 mg group had a 22.7±5.3 point drop in mean HAM-D score from baseline to 12 weeks, while the 600 mg group had a 15.1±7.3 point drop and the placebo group had a 15.2±8.1 point drop (t=2.19, df=17, p=0.043, 900 mg versus placebo; t=2.32, df=14, p=0.036, 600 mg versus 900 mg). When we analyzed the most severely depressed 25% of patients (HAM-D scores of 24 or higher), the time by treatment interaction was nonsignificant (F=1.83, df=6, 56, p=0.11), suggesting that HAM-D scores above 25 are needed to show this effect. Furthermore, when we examined BDI scores in the top quartile, we did not find a time by treatment interaction (F=1.89, df=6, 46, p=0.10).
Assessment of the response rate among the efficacy group (i.e., >50% decline in HAM-D score following the course of treatment) found response rates of 76.5%, 71.8%, and 71.4% among the 900 mg, 600 mg, and placebo groups, respectively. Similarly, remission rates (i.e., HAM-D scores of 8 or lower at the end of the treatment trial) were 41.2%, 43.6% and 40.5% in the 900 mg, 600 mg, and placebo groups, respectively. Among patients in the top quintile of severity, the response rates were 100%, 67%, and 67%, while the remission rates were 86%, 44%, and 50% for 900 mg, 600 mg, and placebo, respectively (χ 2 =3.1, p=0.21; χ 2 =3.2, p=0.20).
DISCUSSION
This study found that 600 mg or 900 mg of nefiracetam administered over 12 weeks of treatment to patients with poststroke major depression led to similar declines in HAM-D scores to placebo. This study also replicated the findings of prior Japanese studies that nefiracetam did not improve cognitive function. When the most severe quintile of depressed patients was analyzed, however, 900 mg of nefiracetam produced significantly greater improvement in HAM-D scores than either 600 mg of nefiracetam or placebo. Similarly, both response rates and remission rates tended to be higher (albeit not statistically significant) among the most severely depressed patients if they received 900 mg of nefiracetam.
Before discussing these findings, the limitations of the study should be acknowledged. First, all patients enrolled in the study had major depression. Whether the response rate would have been the same if patients with minor depression had been included is uncertain. This study, however, did examine a severe form of poststroke depression and would have been expected to show a treatment effect even without the inclusion of patients with minor depression. Second, the patients were predominately Caucasian and in their mid- to late 60s and therefore do not represent the entire demographic cross section of patients with stroke. Thus, the conclusions from this study may not be applicable to all patients with poststroke depression. Third, the placebo patients had significantly greater impairment of cognitive function compared to the other groups and this could have affected the outcome. Even when the analysis was covaried for this difference in initial cognitive function, there was no treatment effect on cognition. Furthermore the lack of correction for multiple comparisons may have led to a chance finding such as this. Fourth, the interviewers and raters in this study were predominately stroke neurologists or nurse interviewers whose background was primarily in assessing physical, not depressive, symptoms and this may have influenced the results. Fifth, because this was a 12-week treatment trial without follow-up, we do not know whether patients who received nefiracetam may have maintained the improvement in their depressive symptoms better than the placebo-treated patients. For example, the treatment study by Fruehwald et al.
38 failed to show a significant effect of fluoxetine for treatment of poststroke depression, but at 1-year follow-up, the patients who received fluoxetine demonstrated significantly lower depression scores and significantly greater improvement in cognitive function compared to patients treated with placebo.
In spite of these limitations, this is the first trial of nefiracetam in the treatment of poststroke depression and it adds to the growing number of randomized, placebo controlled, double-blind treatment studies of this disorder. This is also the largest double-blind treatment study of poststroke depression and the only one to include only major poststroke depression which has been conducted. The response rates for nefiracetam are similar to those reported in other double-blind, placebo-controlled treatment trials. The first treatment trial by Lipsey et al.
5 reported a 100% response rate to nortriptyline among 14 patients who completed the study compared with 33% among 15 patients given placebo. Andersen et al.
7 reported a 61% response rate to citalopram (N=26) compared with 29% placebo response (N=31). Wiart et al.
6 reported 62% response to fluoxetine (N=16) compared to 33% placebo response (N=15). In our latest treatment study,
39 we reported a 77% response rate to nortriptyline (N=16) and a 31% response to placebo (N=17). Thus, the 76% response rate in the present study is not surprising. What was unusual was the 71% placebo response rate. When placebo response rates reach 70% or greater, it is virtually impossible for an active treatment to show superiority. We have found that placebo response rates are significantly higher among poststroke patients who are older and with less severe cognitive impairment (2007 unpublished data), and others have reported greater separation of active and placebo response among more severely depressed patients. Khan et al.,
40 however, reported that higher placebo response rates were found in trials requiring higher pre-randomization depressive symptoms. If less severe depression led to higher placebo response, however, this may explain our finding of a significant treatment effect among those in the top quintile of depression severity.
In summary, although this largest double-blind treatment trial of poststroke major depression did not demonstrate a significant effect of nefiracetam versus placebo, the long-term consequences of nefiracetam on poststroke depression and recovery are still to be determined and its role in the treatment of more severe forms of poststroke depression will require additional investigation.
Acknowledgments
This study was supported by Daiichi Pharmaceutical Co., Ltd., Tokyo, and Prestwick Clinical Inc., Washington, D.C. Dr. Robinson served as a paid consultant for the design of this study and was a member of the Data Safety Monitoring Board. Dr. Clarence Smith was the President of Prestwick Clinical.