C ocaine dependence is a resurgent public health problem that afflicts over 1.5 million American citizens; cocaine is the most prevalent illicit drug detected on blood and urine toxicology screens performed in U.S. emergency rooms.
1 Neuropsychologically, cocaine-dependent patients demonstrate a mild to moderate degree of impairment in such cognitive areas as attention, working memory, learning and memory, and executive functions.
2 –
12 These impairments have been linked in some,
8,
11 but not in all,
7 studies to functional and morphological alterations in (pre)frontal brain regions.
6,
9,
11 Constructional tasks, such as drawing various figures and shapes, are other neuropsychological assessment tools that are particularly sensitive for detection of early or subtle brain damage.
13 Although constructional ability is traditionally assessed within the context of a neurological examination,
13 it is a high-level cognitive function, integrating a complex system of interrelated visual, motor and executive capacities, each of which exhibits a unique role within the context of perceptual/motor behavior. To our knowledge, this has not yet been attempted in cocaine-dependent individuals.
A routine way of assessing constructional ability is through the Copy Figure Test, comprising two- and three-dimensional figures. Such a design offers a more precise interpretation of the findings, that is to say, if a subject inadequately copies both two- and three-dimensional images, it may be inferred that failed performance is not secondary to impaired perception of tridimensionality, and a case for a basic constructional deficit is supported. On the other hand, a control level performance on two-dimensional figures suggests that both eyesight and the ability to translate visual images into kinesthetic concepts and then into motor actions
13 are preserved, and that performance differences are due to three-dimensional processing. In addition, the Copy Figure Test eliminates memory involvement by having the figure to be copied in front of the subject at all times.
13,
14 Furthermore, the test is relatively independent of age, educational status, and intelligence: the ability to copy the figures is fully acquired by the age of 12; older age brackets (ages 40–60) retain normal and unaltered results.
13Consistent with prior reports on neurocognitive impairments,
4,
7,
8,
11,
12 including visuoperceptual deficits,
3,
5,
10 we hypothesized that individuals with cocaine dependence, relative to healthy comparison subjects, would display compromised Copy Figure Test performance. As a second comparison group, we enrolled subjects with marijuana abuse or dependence, who also were not expected to be impaired.
15METHODS
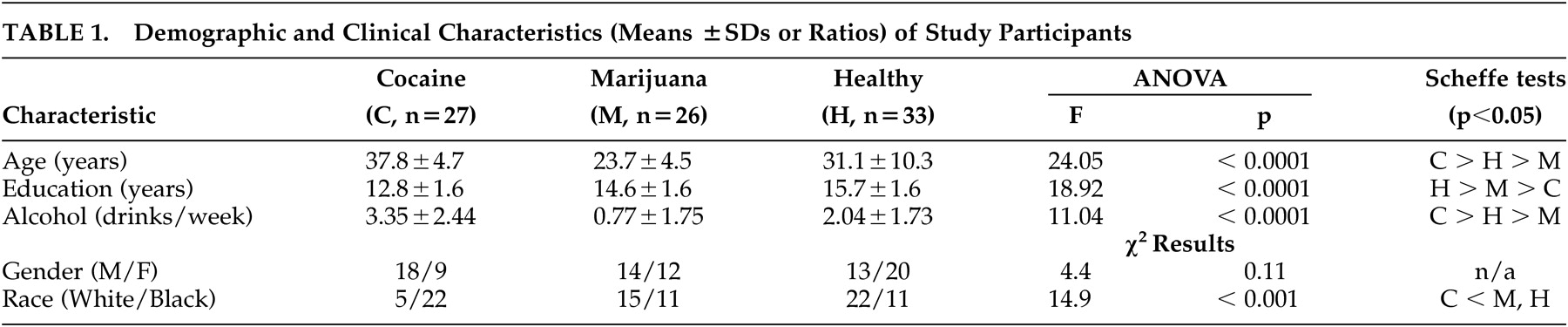
Twenty-seven subjects meeting DSM-IV-TR criteria for cocaine dependence, 26 subjects meeting DSM-IV-TR criteria for marijuana dependence or abuse, and 33 mentally healthy subjects were recruited by advertisement and participated in this study after giving written informed consent. The protocol was approved by the McLean Hospital Institutional Review Board. All subjects were in good physical health and had no history of head injury, loss of consciousness, brain tumor, seizures, or cerebrovascular accident, as determined by neurological screening and by the Cornell Medical Index Health Questionnaire.
16 Education about the effects of the drugs was provided, and referral to drug treatment programs was offered, when appropriate.
Each subject underwent the Structured Clinical Interview for DSM-IV-TR Axis I Disorders.
17 Active cocaine or marijuana use was confirmed by urinalysis; subjects with urine toxicology screens positive for other substances were excluded. Cocaine dependent subjects with a history of any other axis I psychiatric diagnosis, including other substance dependence (but not abuse), were also excluded. Of the 26 marijuana dependence (n=12) or abuse (n=14) subjects, five had a comorbid diagnosis of major depression and four were alcohol dependent. Number of drinks in the week before the study appears in
Table 1 . Cocaine-dependent subjects were not seeking or participating in addiction treatment. They had used cocaine for a mean duration±standard deviation (SD) 15.1±6.9 years, with a frequency of 4.4±2.2 times in the week before the study, and last use was at least 24 hours prior to the study. Marijuana abuse/dependence subjects had smoked this substance for 7.0±5.9 years, with a frequency of 6.6±0.7 times in the week before the study.
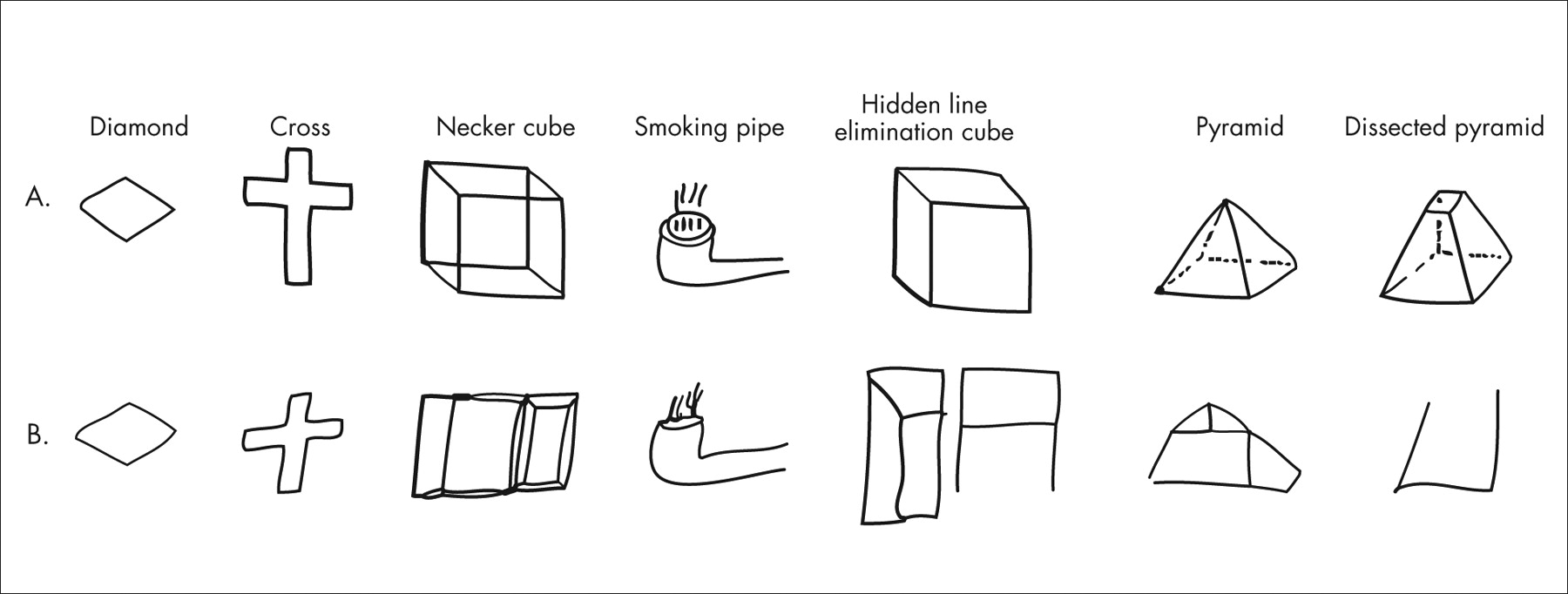
Subjects were instructed to copy each figure (
Figure 1 ) exactly as it looked to them with a pen. They were further told to make only one attempt at each figure, even if they were not completely satisfied with the result, and not to erase any lines or draw any additional lines that did not appear in the original figure. There were no time limits. A dually trained psychiatrist and neurologist (TVG), who not only was blind to the diagnosis but had never seen the subjects, scored each figure on the following Likert-type scale: 0=near perfect reproduction; 1=mild distortion or rotation; 2=moderate distortion or rotation, micropsy, or loss of three-dimensionality; and 3=gross distortion of the basic gestalt (i.e., figure virtually unrecognizable).
Demographic variables were analyzed with analyses of variance (ANOVAs) or chi-square. Overall Copy Figure Test results were analyzed by means of a two-factor analysis of covariance (ANCOVA), with diagnosis (cocaine dependence, marijuana dependence/abuse, or mentally healthy) as a between-subjects factor; figure type (diamond, cross, Necker cube, smoking pipe, hidden line elimination cube, pyramid and dissected pyramid) as a within-subjects factor; and age, education, current number of drinks per week, and race (white or black) as covariates. Additionally, one-factor ANCOVAs were performed separately for each figure, followed by post hoc Scheffe tests when the group effect was significant. The Pearson correlation coefficient was used for correlative analyses. All analyses were two-tailed, and significance was defined as p<0.05.
The rationale for combining marijuana dependence and abuse data was as follows: a higher diagnostic threshold for marijuana dependence due to the lack of a DSM-IV-TR marijuana withdrawal criterion, and no significant differences between marijuana dependence and abuse subgroups on any of the figures (p>0.35).
RESULTS
As presented in
Table 1, cocaine-dependent subjects were significantly older, were less educated, drank more alcohol, and were more likely to be black than both marijuana users and healthy comparison subjects. Marijuana users were significantly younger, were less educated, and drank less than healthy comparison subjects. Gender distribution was not significantly different among the groups.
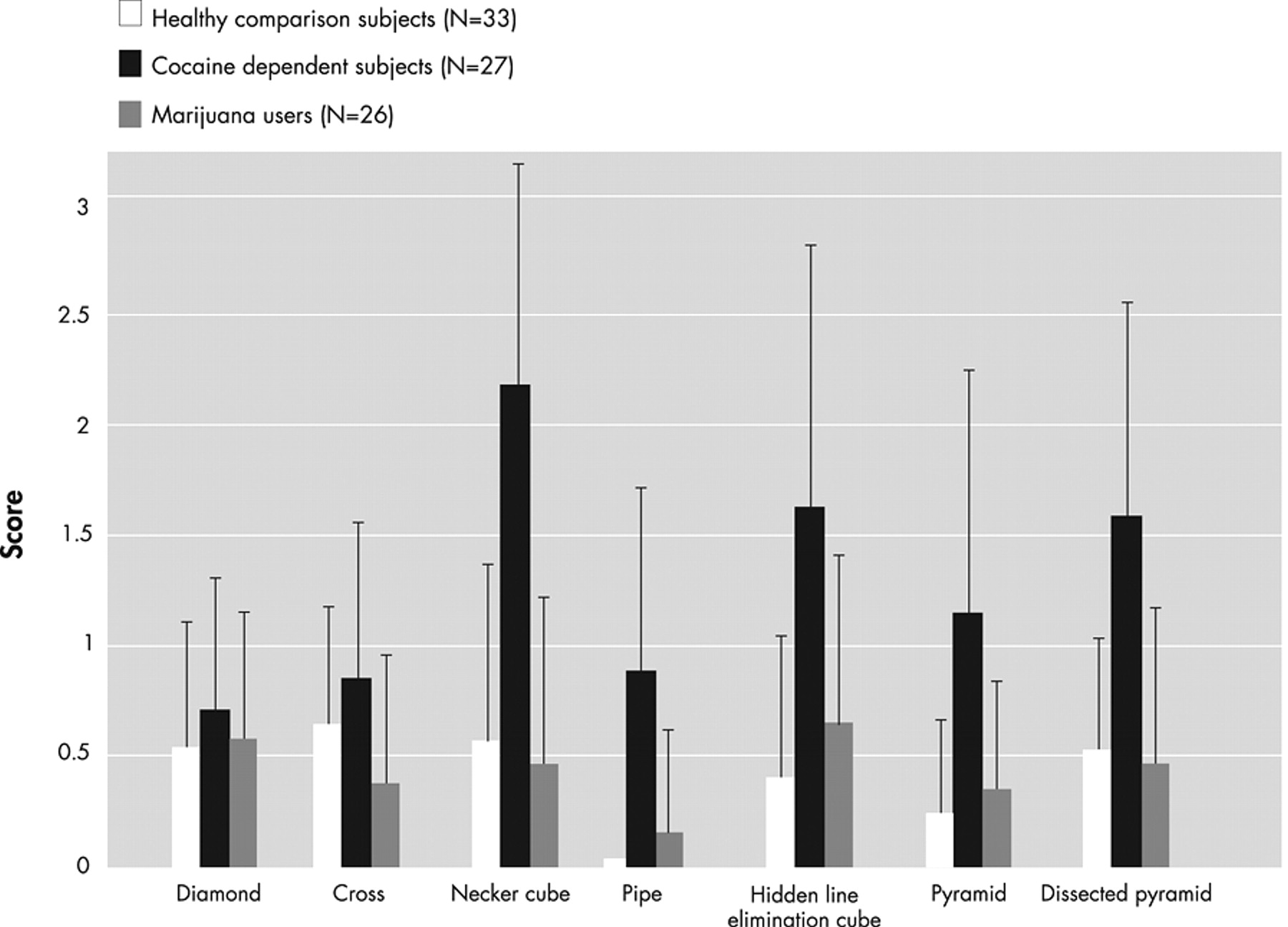
Figure 1 presents examples of mistakes made by cocaine-dependent subjects. The raw group means for each item on the Copy Figure Test are displayed in
Figure 2 . Two-factor ANCOVA yielded a significant diagnosis main effect (F=11.35, df=2, 78, p<0.0001) and a significant diagnosis by figure type interaction (F=2.65, df=12, 468, p=0.002).
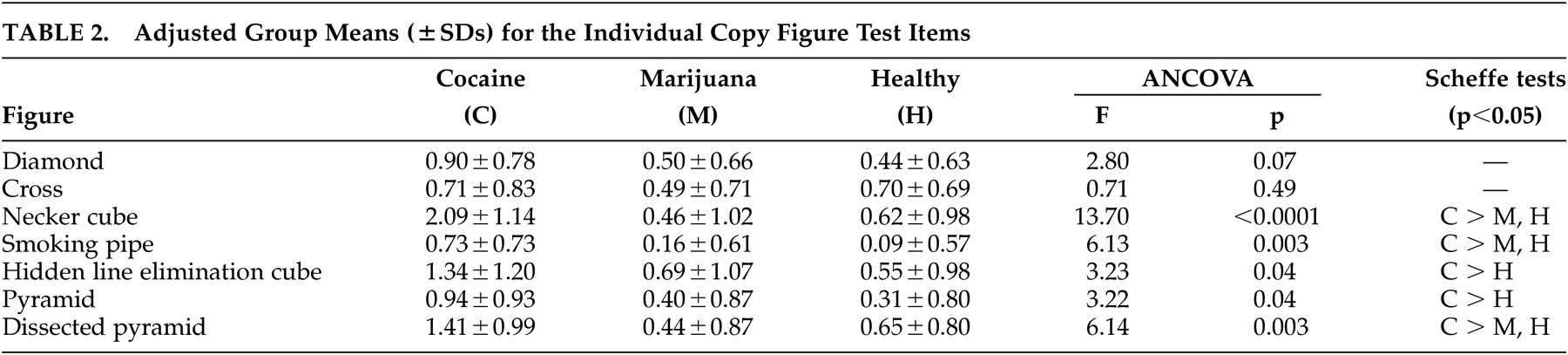
Table 2 presents the adjusted group means for each figure separately, along with results of the univariate ANCOVAs and significant pairwise group differences.
It may be seen that there was no significant group effect on copying two-dimensional figures (diamond and cross). For the three-dimensional figures, there were highly significant differences between cocaine-dependent subjects (worse performance) versus healthy and marijuana-dependent subjects on the Necker cube, smoking pipe, and dissected pyramid, and between cocaine-dependent subjects versus healthy subjects on the hidden line elimination cube and pyramid. The most significant difference between the cocaine group and the other two groups was on the Necker cube, which is characterized by ambiguous front-back orientation necessitating visuospatial ability to shift attention between two equally plausible figural spatial representations.
18 Eighteen cocaine-dependent subjects (67%), one healthy subject (3%), and zero marijuana group subjects scored 3 (gross distortion of the basic gestalt) on at least one figure. There were no significant differences between healthy versus marijuana group subjects on any figure; indeed the performances of these two groups were highly comparable.
No correlations that approached statistical significance were found between age (r=0.04, df=25, p=0.85), education (r=−0.29, df=25, p=0.14), and the average Copy Figure Test score in the cocaine-dependent group, making these unlikely sources of diminished Copy Figure Test scores in this group, which were statistically adjusted for these factors.
DISCUSSION
To our knowledge, this is the first study to specifically assess constructional ability (i.e., capacity to draw two- and three-dimensional figures) in drug-dependent individuals. The major finding is that, notwithstanding the normal copying of two-dimensional figures, cocaine-dependent subjects, but not healthy or marijuana group subjects, displayed substantial impairment in copying three-dimensional figures. Sixty-seven percent of cocaine-dependent subjects, but almost no healthy or marijuana group subjects, showed a gross distortion of at least one figure, which is “almost pathognomonic” of brain damage.
13In addition to its acute detrimental medical consequences, chronic cocaine use adversely affects its primary target organ, the brain,
19 with lacunar
20 and diffuse tissue reductions
21 as well as changes in cerebral perfusion and metabolism.
22 In light of this, it is tempting to conclude that the origin of the figure copying impairments found in this study is cocaine damage to the brain. However, it is also possible that a preexisting genetic
23 or acquired risk factor, which is manifest in impairment in copying figures, is imparting vulnerability for cocaine dependence in a similar manner to subtle neurological dysfunction in attention deficit hyperactivity disorder
24 as well as in victims of childhood abuse.
25 Resolving the origin of this impairment could require prospective and/or twin studies.
There is consistent clinical
26 and postmortem
27 evidence for a relationship between cocaine use and fatal injuries, with cocaine’s emergence as one of the five major causes of mortality among young people.
27 Cocaine-induced agitation and paranoia may certainly account for some violent deaths,
27 but the drug’s acute beneficial effects on attention and vigilance render it an unlikely direct reason for motor vehicle accidents.
28 Difficulties with spatial integration (along with other neurocognitive functions) as a consequence of long-term sustained cocaine use may be an alternate explanation, considering that decreased performance on copying a cube predicts motor vehicle crashes.
29Several brain regions influence the drawing of three-dimensional figures, the most important of which appear to be parietal.
13 Specifically, patients with right parietal lobe lesions appear especially unable to reproduce the basic gestalt of such figures.
13 Seeking to tackle methodological limitations of early research on cortically damaged patients, Inui and colleagues
18 used functional MRI during the visual processing of cubes versus two-dimensional overlapping squares in a sample of healthy subjects and found symmetrical parietal area activations.
Ventral striatum and related mesolimbic dopaminergic circuitry are traditionally considered to be the key component of the reward system involved in cocaine dependence, and it is commonly hypothesized that cocaine-induced changes in the mesolimbic pathways underlying motivational processes are responsible for transforming regular drives into heightened incentive salience assigned to drugs or drug-related cues, such as incentive sensitization, clinically evident as craving.
30 However, recent work in primates suggests a novel factor in the mechanisms underlying incentive sensitization and craving by implicating the parietal cortex in the control exerted over striatal signals of salience via integration of visuospatial, motor, and cognitive (e.g., hedonic value and categorical boundaries) inputs.
31,
32 In addition to these theoretical considerations, an abundant clinical literature demonstrates parietal cortex changes in the context of chronic cocaine consumption.
23 There is a need to focus on this important region and its role in the pathophysiology of cocaine dependence.
A limitation of this study is that group differences in age, education, race, and alcohol use required us to perform statistical adjustments for these potentially confounding variables. Future studies should attempt group matching on these factors as far as possible within subject recruitment exigencies.
It is possible that the cocaine-dependent subjects may have underreported the extent of concomitant alcohol use,
33 which is common in these patients. Moreover, this study only quantified alcohol use during the previous week. Prior research has implicated alcohol in spatial disturbances.
34 However, none of the cocaine subjects fulfilled DSM-IV-TR criteria for past or current alcohol dependence. Additionally, it is unlikely that relatively mild alcohol consumption worsened cocaine-dependent subjects’ responses. If anything, alcohol tends to improve, or at least not to worsen, cognitive and visuospatial function in this group,
19,
20,
35,
36 perhaps via amelioration of cocaine’s acute vasoconstrictive effects.
37,
38 Nevertheless, a more thorough quantification of this important variable over a longer time period prior to testing, using instruments validated for this purpose, should be incorporated in future studies since chronic use of both cocaine and alcohol causes an enduring neurocognitive impairment, which ultimately might have an additive effect.
Finally, the paradigm employed does not allow us to firmly conclude that a higher-level constructional impairment is not implicated in the poorer three-dimensional figure copying observed in the cocaine-dependent subjects. Answering this question would require disentangling this component via an exclusively visual-processing paradigm that did not employ motoric output.
The present findings offer a new perspective on the pathophysiology of cocaine dependence. It should be noted that the test employed here is but one component of a 45-item battery of tests for neurological soft signs available for neuropsychiatric research.
39,
40 Even though the Copy Figure Test has proven to be the most sensitive tool in our prior work with posttraumatic stress disorder patients,
39,
40 the preset cocaine findings may not necessarily be specific to the deficits uncovered by the Copy Figure Test and could generalize to other neurological and/or cognitive impairments. Therefore, it would be revealing to perform a follow-up study that employs the full battery.
Acknowledgments
This work was supported by grant DA # 017959 (IE) from the National Institute on Drug Abuse.