A modern apparatus of repetitive transcranial magnetic stimulation (rTMS) was developed in Great Britain in 1985.
1 Since then, rTMS has been utilized to provide noninvasive brain stimulation for research and treatment purposes. The most frequent application of rTMS in psychiatry has been as a treatment for major depression, and the first controlled treatment study was published in 1996.
2 Although a large number of studies have suggested that rTMS is efficacious against depression, a recent review found that the efficacy can be quite variable.
3 This may be due to the fact that the etiological factors underlying major depression are heterogeneous, causing some forms of depression to respond better to rTMS than others. Repetitive transcranial magnetic stimulation has already been approved as a standard treatment procedure for depression in some countries such as Canada and Israel; however, it has only recently been approved by the Food and Drug Administration in the United States.
Predictors of response to different antidepressant treatment modalities are a growing area of clinical research interest. Higher than normal activity in the rostral anterior cingulate cortex (ACC) has been shown to predict response to antidepressant medication in patients with major depression.
6 –
9 High theta power in the rostral ACC has also been found to predict response in a similar population.
10 This same group found that metabolism and theta activity are linked in this region.
11 However, functional abnormalities in this region do not change in response to antidepressant treatment. Treatment response to antidepressants has been correlated to change in the subgenual region of the ACC.
12 It is unclear whether this is a normalization of high metabolism or a resetting to a new lower level of metabolism in this region.
13 The subgenual ACC has become a target for deep brain stimulation, which has already shown efficacy in treatment-resistant depression.
13 In the present study, we hypothesized that abnormal theta activity occurs within the rostral and/or subgenual regions of the ACC in patients with vascular depression and may predict an antidepressant response to rTMS.
MATERIALS AND METHODS
Participants
Sixty-five participants with medication resistant vascular depression underwent EEG recordings before and after a course of rTMS. They had been recruited from both outpatient and inpatient clinics of the Department of Psychiatry at the University of Iowa Hospitals and Clinics and the Iowa City Veterans Affairs Medical Center, as well as through advertisements in metropolitan areas in the state of Iowa, between May 2002 and October 2006.
The inclusion criteria for this study were age between 50 and 90 years old; major depressive disorder (as diagnosed by the DSM-IV-TR using the Present State Examination
14 modified to identify DSM-IV-TR) with onset after age 50 and a score above 14 on the 17-item Hamilton Depression Rating Scale (HAM-D); a history of stroke or at least three of the following cardiovascular risk factors: (a) arterial hypertension, (b) diabetes mellitus, (c) obesity, (d) hyperlipidemia, or (e) smoking; and unresponsiveness to at least one antidepressant given at an adequate dose and length with no compliance issues (as assessed by the
“ Antidepressant Treatment History Form”).
15 Exclusionary criteria included left frontal cortex lesion or hemorrhagic stroke to avoid rTMS induced seizure; life-threatening physical illness; neurodegenerative disorder; clinical dementia (Clinical Dementia Rating Scale score >0.5)
16 ; severe aphasia (i.e., inability to complete part 1 of the Token Test)
17 ; suicidal thought, plan, or delusion; substance abuse within the prior 2 years; prior seizure or traumatic brain injury; and any metallic device which would preclude an MRI scan. If the participants were taking antidepressants, these were discontinued for at least 2 weeks before rTMS (except for fluoxetine where a minimum discontinuation of 3 weeks was required). Written informed consent was obtained from all participants. This project was conducted as approved by the Institutional Review Board at the University of Iowa Hospitals and Clinics and Iowa City Veterans Affairs Medical Center.
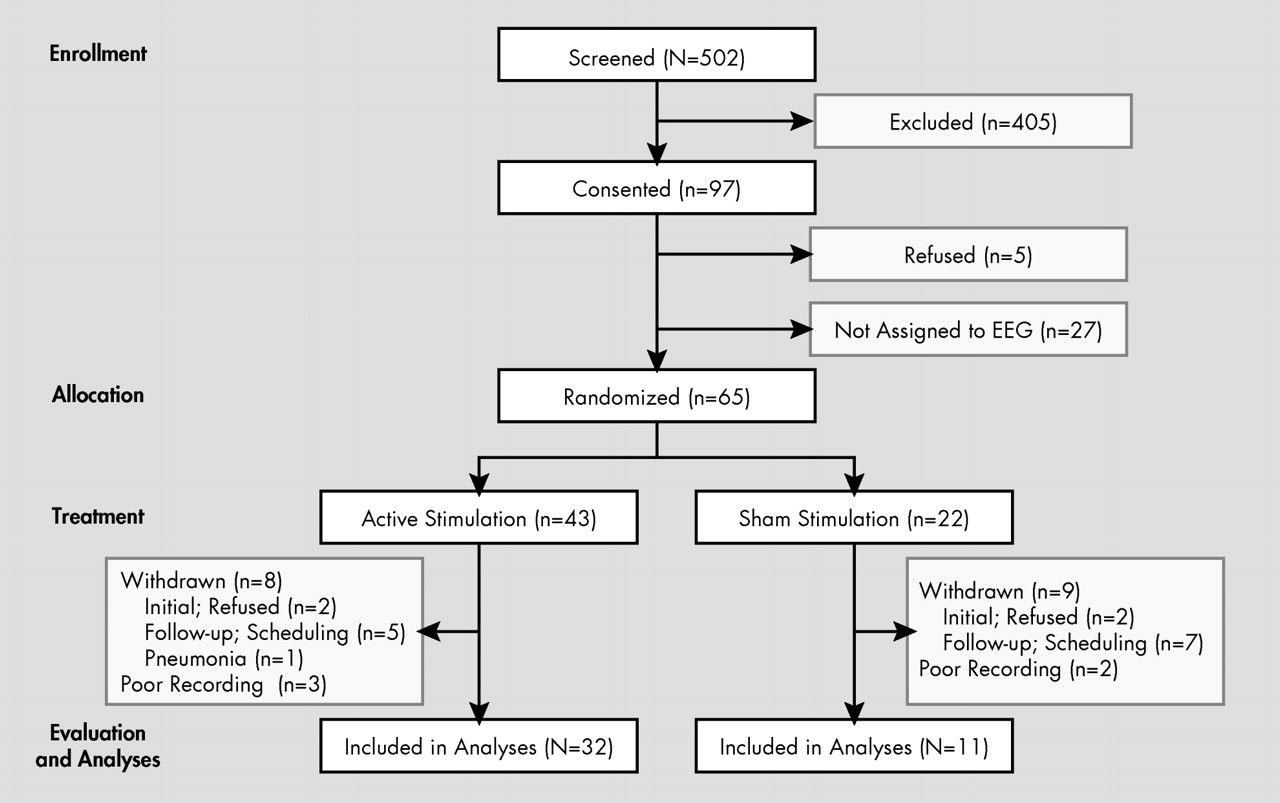
Initially, a total of 502 participants were screened; of these, 97 met our inclusion criteria and agreed to enroll in this study (
Figure 1 ). Among these 97 participants, five withdrew before treatment. Among the remaining 92 participants, 65 who had rTMS after July 2003 were given an EEG evaluation. Among these, 43 participants were randomly assigned to active rTMS; 12 received a total of 12,000 stimulations whereas 31 had 18,000 stimulations. Those two groups were combined into a single group for data reduction, because there were no significant differences in their demographic background, clinical presentation, or primary outcomes in post hoc analyses. Among these remaining 43 participants, two refused an initial EEG examination and six participants did not return for their follow-up evaluation; thus, a total of eight participants were withdrawn from the active treatment group. Of the remaining 35 participants, three were excluded due to poor quality recording. Thus, the final number in the active treatment group was 32. Among 22 participants assigned to sham stimulation, two refused an initial EEG recording and seven participants did not have a follow-up evaluation due to scheduling difficulties. Among the remaining 13 participants, two had a poor quality EEG. Thus, the final number in the sham treatment group was 11 (
Figure 1 ). All participants successfully completed the full 2-week course of rTMS with no significant adverse effects. There were no significant differences in demographic background between participants who completed the study and those who were withdrawn.
Lesion Location
Magnetic resonance imaging (MRI) scans were obtained for all participants except two due to scheduling problems. Scans were evaluated for anatomical location and volume of the hyperintensities by radiologists who were blind to the clinical findings and further processed using locally developed BRAINS2 software (www.psychiatry.uiowa.edu/mhcrc/IPLpages/BRAINS.htm). The validity of this methodology has been demonstrated in previous publications.
18Neuropsychiatric Evaluations
The 17-item HAM-D
19 was employed before and within 1 week after a 2-week course of rTMS. HAM-D constitutes a valid measurement of depressive symptoms among participants with vascular depression.
20 A decrease of at least 50% in HAM-D scores was defined as “response” to rTMS. “Remission” was defined by a decrease of at least 50% on HAM-D and a final HAM-D score below 9. The HAM-D was given by trained research assistants who were blind to the clinical/treatment information. The participants were also given a Mini-Mental State Examination
21 at the same visit. A full description of the neuropsychiatric evaluations has been published elsewhere.
22rTMS Procedure
Localization of the Stimulation Site
rTMS procedure was described in detail in our prior publication. Briefly, it involved localization of the stimulation site in the left dorsolateral prefrontal cortex using Brainsight Frameless (www.magstim.com/brainsight/). A landmark was picked up on the cortex corresponding to the midpoint of the middle frontal gyrus. Finally, the Talairach coordinates of the landmark in both the anterior-posterior and dorsal-ventral directions were checked. This landmark, and thus the stimulation site, lay within the conservative range of Talairach coordinates for Brodmann’s area 46 as described elsewhere.
23Stimulation Parameters and Monitoring
rTMS was performed using a Magstim Super Rapid Stimulator (Jali Medical, Inc.) with 70 mm figure-8 coils. The participants were randomly assigned to three groups; two groups received active rTMS over the left dorsolateral prefrontal cortex at a frequency of 10 Hz and an intensity of 110% of the motor threshold during 6 seconds (the motor threshold was determined as the lowest stimulator intensity that induced a motor evoked potential of at least 50 mV in at least five of 10 trials using the first dorsal interosseous muscle). A total of 20 trials were given (separated by 1 minute pauses) per session. Treatment was administered over 10 days for a total cumulative dose of 12,000 pulses (n=12) or 18,000 pulses (n=31). Sham stimulation was performed using a sham coil, which was identical to the stimulating coil with vibration and noise (albeit without actual cortical stimulation).
EEG Recording
The EEG recordings were obtained before and within 1 week after a series of rTMS. EEG was recorded for at least 20 minutes using an electrode cap with ten electrodes from 19 scalp locations based on the international 10/20 system (FP1/2, F3/4, C3/4, P3/4, O1/2, F7/8, T3/4, T5/6, Fz, Cz, Pz), using linked ears as a reference. Electrode impedances were under 5kΩ. Data were collected digitally with a sampling rate of 200 Hz, and a digital band-pass filter (1–70 Hz) was used offline.
For all participants, EEG acquisition occurred with a Neurofax system (Nihon Kohden). At least 3 minute segments of EEG were recorded during an eyes-closed resting condition. Each EEG recording was examined to remove artifacts by using NeuroGuide software (http://www.appliedneuroscience.com), which was further visually confirmed by neuroscientists who were blind to the clinical information (KN, TY). Nonoverlapping, artifact-free 60 sec epochs were selected from each recording. Split-half and test-retest reliability tests were conducted and only records with >95% split-half reliability and >90% test-retest reliability were entered into the following analyses.
LORETA Computation
All EEG epochs were further analyzed with low resolution electromagnetic tomography (LORETA-KEY software package; www.uzh.ch/keyinst/loreta), which interfaces with NeuroGuide. From the scalp-recorded electrical potential, LORETA computed the three-dimensional space electrical potential distribution. LORETA utilizes a whole-brain approach, and computations were restricted to cortical gray matter. The spatial resolution was 7 mm, and the solution space contained 2,394 voxels with spectral resolution of 1 Hz.
Analyses were conducted in a stepwise fashion. All available EEG epochs were subjected to a whole brain cross-spectrum analysis for the following EEG bands: delta (1–3 Hz), theta (4–7 Hz), alpha (8–12 Hz), beta (13–30 Hz). We further divided the theta band into low (4–5 Hz) and high (6–7 Hz) frequencies based on the fact that Pizzagalli et al.
10 found that high theta activity (6.5–8 Hz) predicted a response to antidepressant medication. LORETA computed current density as the linear, weighted sum of the scalp electrical potentials and squared this value to yield power of current density for individual frequencies in each voxel. The LORETA solution was normalized to a total power of 1 and log-transformed for each band.
Region-of-interest clusters were analyzed for the regions corresponding to the rostral and subgenual ACC as provided by the LORETA legend, which allows identification of the average power for each frequency of every voxel to be analyzed separately. There are 188 voxels for the entire ACC. The subgenual region that corresponded to the entire BA25 was made up of 29 voxels (total volume 9.95 cm
3 ), while the rostral region was made up of 52 voxels (17.84 cm
3 ). Compared to a normative database, LORETA units in these ACC subregions at baseline were analyzed by cluster t statistics. For comparison to the nondepressed, normal control population, an age-adjusted normative module within NeuroGuide was employed which was based on 625 individuals from 2 months to 82 years of age who met specific clinical standards including no history of neurological disorders or behavioral disorders as well as attainment of an age-appropriate level of education.
24 LORETA is quite accurate when there are no artifacts in the data as evidenced from over 500 peer reviewed articles, which have found that LORETA compares favorably to the more classical functional imaging methods, such as PET and fMRI.
25 In order to solve the ill-posed inverse problem (i.e., how to determine electric sources based on scalp recorded surface data), LORETA makes a “smoothness assumption” that neighboring voxels should have maximally similar activity. The LORETA algorithm allows for correct localization at a special resolution to within 1 voxel or 7 mm resolution on average.
26 Several studies provided validation for EEG-based LORETA analysis using MRI,
27 fMRI,
28 or PET.
10Hyperintensity Lesions
Among the 41 participants who completed a brain MRI scan, five scans were not analyzed due to bad segmentation or poor imaging quality. Among the remaining 36 participants, 27 showed multiple hyperintensities. There were no significant differences between the responders and the nonresponders in the locations or total volume of hyperintensity lesions. Full descriptions of the imaging analyses have been published previously.
22 Only 8% of patients previously had clinical stroke (no significant difference among the treatment groups).
Other Statistical Analyses
Between-group comparisons were carried out using means, standard errors, and analysis of variance (ANOVA). Frequency distributions were compared using chi-square tests or, where appropriate, Fisher’s exact test. All tests were two-tailed; p value was set at 0.05.
DISCUSSION
The present study showed that nearly half of the drug-resistant vascular depression participants responded to rTMS, whereas virtually none of the participants who received sham rTMS responded. This study also found that increased pretreatment low-theta power in the subgenual ACC was associated with the antidepressant response from rTMS. To our knowledge, this is the first study to identify a prospective predictor for antidepressant response to rTMS among participants with treatment-resistant vascular depression using EEG analyzed with LORETA.
The efficacy of rTMS for depression has been shown to be quite variable and only slightly better than the sham condition in some studies (e.g., 37% response to active treatment versus 20% to sham).
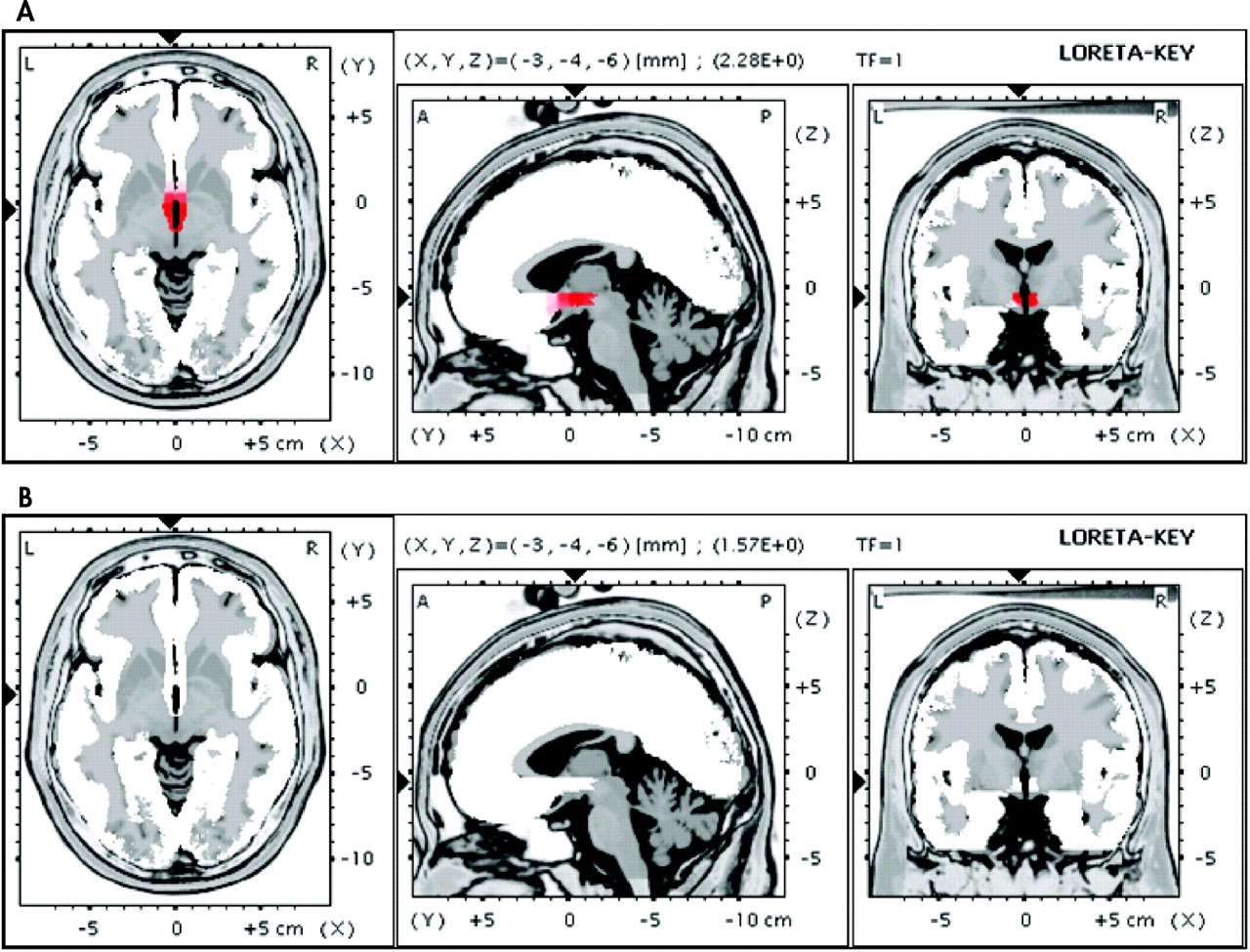
3 A high response rate to sham stimulation has been one of the most significant issues affecting the consideration of efficacy of rTMS. Given the relatively high efficacy of active rTMS (43.8%) and the low response rate to sham (9.1%) in our study, vascular depression seems to be a quite appropriate population to be treated by rTMS. It is crucial, however, to further define appropriate participants who are likely to respond to rTMS. Our analyses (
Figure 2, panel A) revealed that responders displayed, on average, electrophysiological brain activity in the subgenual ACC (defined as 4–5 Hz power) that was 2.28 standard deviations above the control, whereas the nonresponders (
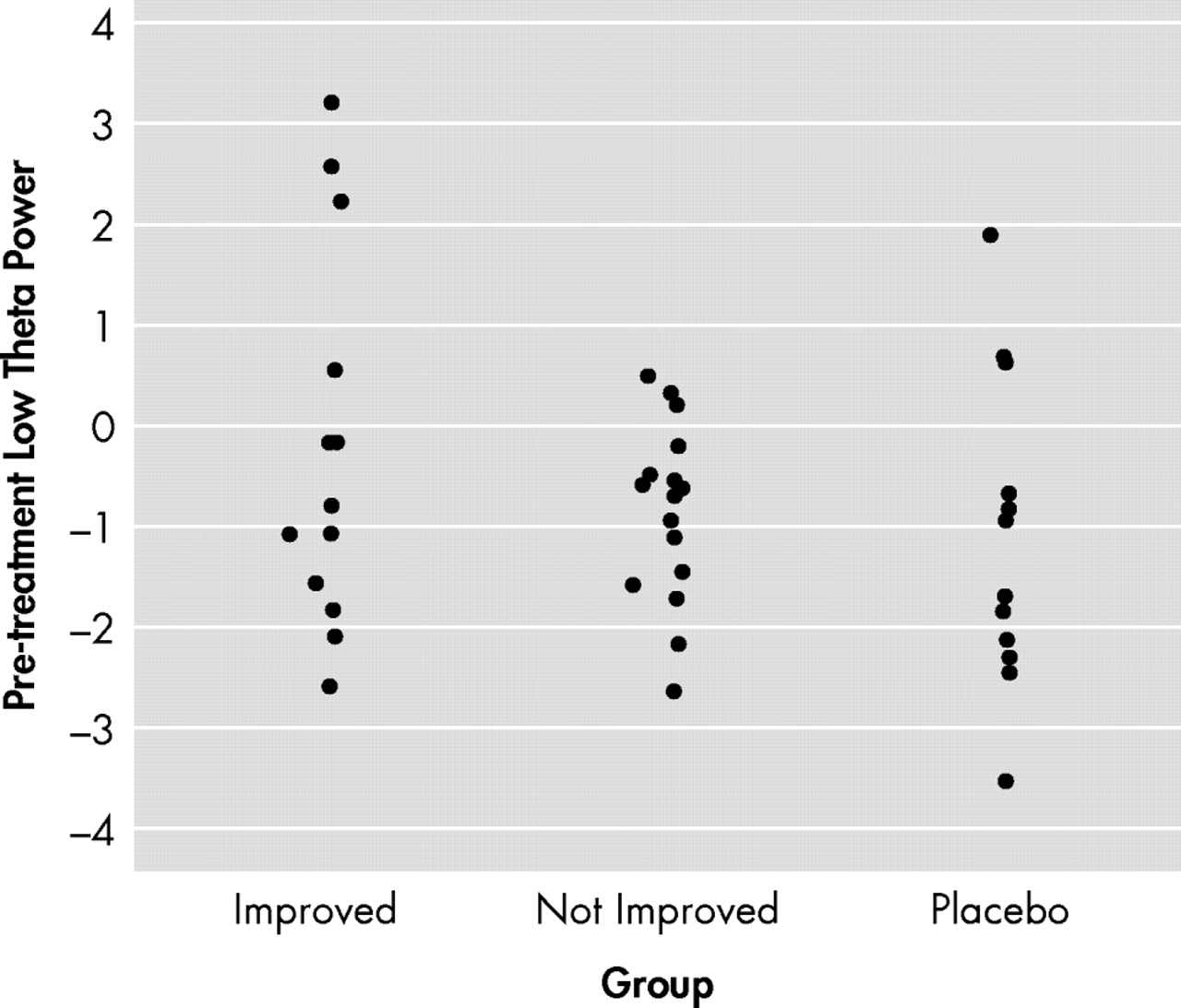
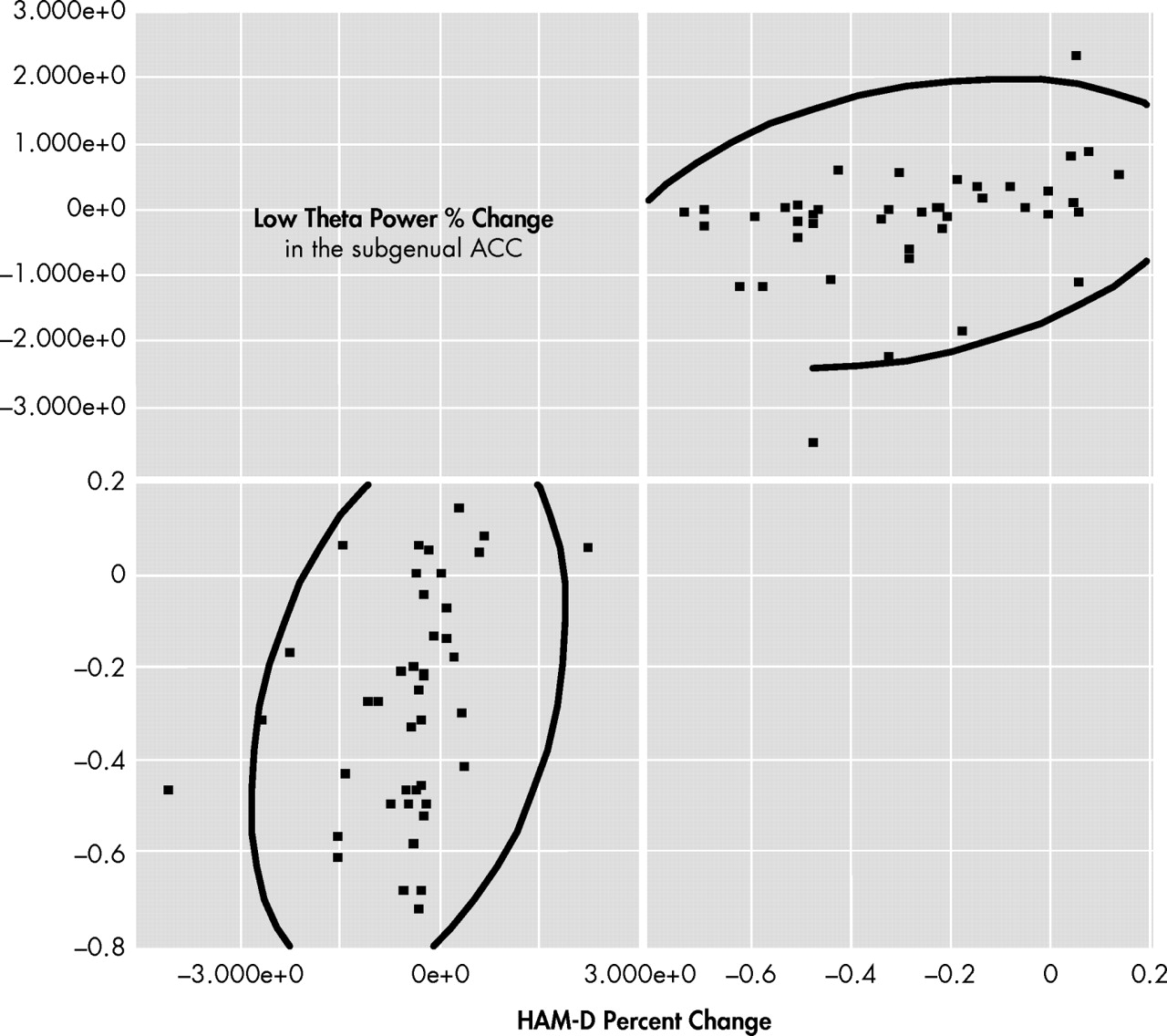
Figure 2, panel B) did not display any statistical differences. Region-of-interest analysis of the subgenual ACC showed a positive and significant correlation between percent changes in low-theta power and total HAM-D scores among the actively treated participants (Spearman ρ=0.45, p<0.01;
Figure 4 ). In addition, before the rTMS stimulation, there was a positive and moderate correlation between low-theta power in the subgenual ACC and total HAM-D score among all the participants (Spearman ρ=0.38, p=0.01). These correlations suggest a significant role for the subgenual ACC in the development of depression among patients with vascular diseases.
Functional brain imaging has played a primary role in the search for predictors of response to different treatment modalities for major depression, and EEG with LORETA can be used in a similar way.
29 The brain region with the greatest prospect of pretreatment prediction is the rostral ACC.
6 –
8 Metabolic and electrophysiological activity changes in depression are linked in this particular part of the brain.
10 Although the rostral ACC is anatomically connected to the subgenual ACC, these regions are cytoarchitecturally distinct.
9,
30 –
32 The subgenual ACC is considered a more archaic region than the rostral ACC; has extensive connections with other parts of the brain such as the amygdala, raphe nuclei, and brain stem autonomic nuclei; and is involved in emotional processing, monoaminergic neurotransmitter release, and autonomic regulation.
33 While abnormal function has been found in both the rostral and the subgenual ACC in major depression, only the subgenual ACC has been associated with a reduction in volume,
31,
34 and this may represent the core abnormality of treatment resistant depression.
35 Although elevated activity in the rostral ACC has been shown to predict response to pharmacological treatment,
9,
10 response to antidepressant treatment has been shown to be primarily mediated by the subgenual ACC. A recent study by Kitou and Koga.
36 reported that active rTMS decreases regional blood flow in the subgenual ACC and is associated with efficacy, and similar findings have been reported using antidepressant medication
12 and deep brain stimulation.
13 The subgenual ACC is also known to become activated during normal sadness
37 and may be overly active in patients with major depression. These findings suggest that higher than normal activity in this specific brain region predicts response to different treatment modalities.
38 Although rTMS does not stimulate the subgenual ACC directly, the blood flow studies of Kitou and Koga.
36 show several brain regions being activated from rTMS, suggesting current spread from the dorsolateral prefrontal cortex through neighboring structures known to have significant connections to the subgenual ACC.
32 Compared to previous publications, the presentation of regional brain abnormalities was slightly different in our participants who eventually responded to the treatment, such as regions (rostral or subgenual ACC) or changes in activity (increase or decrease), which may be attributable to the subtype of depression and ability to respond to different forms of antidepressant therapy.
Limitations of this study include the fact that the majority of participants were Caucasian and from a relatively higher socioeconomic status that may not be representative of the depressed population as a whole. Second, attrition rates were high, and we were unable to obtain an equal amount of evaluations in the sham group due to study design. Third, LORETA has been utilized by multiple experienced groups in this field of research over the past 10+ years; however, the use of a standardized normative database is a relatively new modality for confirming differences with healthy comparison subjects. Similarly, there is currently no database or information about theta power in the ACC in subjects who have had similar vascular problems but without symptoms of depression. Additionally, there was no further follow-up evaluation for recurrence of depression, no qualitative evaluation for the sham stimulation, no voltage data applied, and no functional neuroimaging provided. Lastly, although vascular depression is considered a distinct diagnostic subtype, there are still no universally accepted diagnostic criteria for clinical vascular depression.
Since abnormal generation in a part of the theta band was found in vascular depression participants who responded best to rTMS, these findings suggest that normal theta activity in the subgenual ACC is an important aspect of emotion regulation, which when disrupted is linked to depression. Larger scale prospective rTMS studies that also include functional imaging to localize the path of rTMS current simulation are necessary to validate the findings in this study. Improved efficacy of rTMS, however, might be achieved among patients with vascular depression who show increased 4–5 Hz theta power in the subgenual ACC at baseline.
Acknowledgments
Presented in part at the American Psychiatric Association Annual Meeting in Washington, DC, May 5, 2008 (the 2007-2008 APA/Lilly Resident Research Award).
We thank Teresa Kopel, M.A., Stephanie Rosazza, B.S., Jose A. Abreu, J. Todd Kosier, B.S., and Michael C. Brumm, B.S., for participant recruitment and EEG collection. We are indebted to the Department of Psychiatry at the University of Iowa Hospitals and Clinics and the Department of Psychiatry at the Iowa City Veterans Affairs Medical Center in Iowa City for allowing us to access the participants for inclusion in the study. This study was supported in part by National Institute of Mental Health R01 grant MH63405 (RGR).