Primary progressive aphasia (PPA) is a neurodegenerative disorder characterized by an isolated and gradual dissolution of language function.
1 Cases of PPA-like aphasias along with behavioral disturbances were first noted by Pick in 1892,
2 but it was almost 100 years later, in 1982, when PPA was more adequately defined by Mesulam.
1 For a diagnosis of PPA, memory, visual processing, and personality of the individual are relatively intact, and language dysfunction is the salient feature for about the first 2 years of the disease.
3–6 Subsequently, as the disease progresses, other cognitive domains may become impaired, leading to PPA-plus, although the language dysfunction remains the prominent impairment.
7 This particular isolation of language dysfunction is what distinguishes PPA from behavioral-variant frontotemporal dementia (bvFTD)
8,9 and typical forms of Alzheimer's dementia.
7 PPA is classified into agrammatic (PPA-G), semantic (PPA-S), and logopenic (PPA-L) variants.
10,11 Also, each PPA variant has a different probability of association with Alzheimer's disease versus frontotemporal lobar degeneration.
10Even though there is a growing interest in the investigation of the nosology, neuropsychology, and neuropathology of PPA, little is known about its neuropsychiatric aspects.
8,12 Banks and Weintraub compared the frequency of neuropsychiatric symptoms in PPA with that of bvFTD.
13,14 We conducted a similar study, but we sought to examine whether neuropsychiatric symptoms (NPS) occur in PPA over and above that expected in the general population by conducting a case–control study in which the controls were sampled from an ongoing population-based study. Our study is intended to contribute to the developing literature on the neuropsychiatric aspects of PPA. This is important because NPS may be clinical markers for various phases of the underlying neurodegenerative disorder manifesting with PPA and subsequently in PPA-plus.
13METHOD
The study was approved by Mayo Clinic and Olmsted Medical Center Institutional Review Board (IRB).
Participants
This is a case–control study, in which 55 cases of PPA were individually matched by age, sex, and educational level at a ratio of 1:2, with 110 cognitively normal persons sampled from an ongoing population-based study.
Definition of Cases
A consensus panel of behavioral neurologists, neuropsychologists, and nurses made a diagnosis of PPA on the basis of Mesulam's criteria: insidious onset and gradual progression of primary language problem such as word-finding, object-naming, or word-comprehension impairments as manifested during conversations or as assessed through formal neuropsychological testing; all limitations of daily activities are attributable to the language impairment within about the first 2 years of the illness; also, no significant impairment in other cognitive domains within the first 2 years (acalculia and ideomotor apraxia may be present within the first 2 years of the illness), absence of “specific” causes, such as stroke or tumor, as ascertained by neuroimaging; other domains possibly affected after the first 2 years, but language remains the predominant cognitive impairment.
4Definition of Controls
The Controls are participants in the Mayo Clinic Study of Aging, which is a population-based study of aging and mild cognitive impairment (MCI) in Olmsted County, MN.
15 Elderly persons are recruited using stratified random sampling from the target population of nearly 10,000 elderly individuals living in Olmsted County on the prevalence date of October 1, 2004. The sampling involved equal allocation of men and women in two age-strata: 70–79 years old and 80–89 years old. Each participant in the Mayo Clinic Study of Aging underwent the following three baseline face-to-face evaluations: 1) a neurological evaluation by a behavioral neurologist; 2) a risk-factor assessment by a nurse or study coordinator; and 3) neuropsychological assessments of memory, executive function, language, and visuospatial skills. The tests were administered by a psychometrist, and the results were interpreted by a neuropsychologist. The interview by the nurse or study coordinator includes administration of the Clinical Dementia Rating Scale (CDR)
16 to the participant and to an informant, as well as collection of neuropsychiatric data. An expert consensus panel of physicians, nurses, and psychologists meets on a weekly basis and reviews the neurological, nursing, and neuropsychological data and classifies a person as cognitively normal or not.
15Measurement of Neuropsychiatric Symptoms (NPS)
The NPS of all Cases and Controls were measured by the Neuropsychiatric Inventory (NPI), with the exception of one Case and two Controls whose NPS were measured by the Neuropsychiatric Inventory Questionnaire (NPI-Q). The NPI and NPI-Q measure 12 behavioral domains, including depression, apathy, irritability, agitation, anxiety, disinhibition, delusions, aberrant motor behavior, euphoria, hallucinations, night-time behaviors, and appetite/eating change. Both NPI and NPI-Q have been shown to be reliable and valid scales.
17,18An experienced research nurse or psychometrist administered the NPI to a spouse or other informant for all study participants. The information was gathered through a series of screening questions for each of the 12 behavioral domains. If the informant answered a question affirmatively, the specific behavior was further queried for frequency (range: 1 to 4) and severity (score: 1 to 3). The product of frequency and severity gave the composite score (maximum: 12) for each of the 12 behaviors. Adding the total scores of the 12 behavioral domains yielded the total NPI score.
Statistical Analysis
We compared the neuropsychiatric profiles of Cases and Controls by using odds ratios (OR) and the corresponding 95% confidence intervals (95% CI). The OR and 95% CI were computed by multivariable logistic-regression analysis. Statistical testing was done at the conventional two-tailed alpha level of 0.05. All analyses were performed with SAS (Cary, NC). The Cases (N=55) and Controls (N=110) were individually matched by age, sex, and educational level, hence, by design, there was no difference between Cases and Controls in these three variables. Functional ability as measured by CDR was entered in the model as a covariate. Therefore, the design and analysis of the study ensured that the differences in the frequency of NPS between Cases and Controls were not due to age, sex, education, or functional status.
RESULTS
Consistent with the matched Case–Control design, there was no significant difference between PPA and cognitively normal persons in age, sex, or education. The median age of the PPA group was 70.5, and that of the cognitively normal group was 70.8. The sex distribution was also identical in both groups (30/55 cases of PPA [54.5%] and 60/110 of the cognitively normal persons [54.5%] were men). The median education level for both Case and Control subjects was 14 years. There was no difference between Cases and Controls when education was also dichotomized at 12 years (≤12 years versus >12 years of education). As expected, there was a significant difference in median CDR score between PPA Cases and Controls (p<0.001), which was 0.5 for PPA and 0 for Controls. The median BNT score was 21.5 for PPA and 55 for Controls. We chose to use the median because it is a more robust measure of central tendency than the mean, which is quite sensitive to outliers.
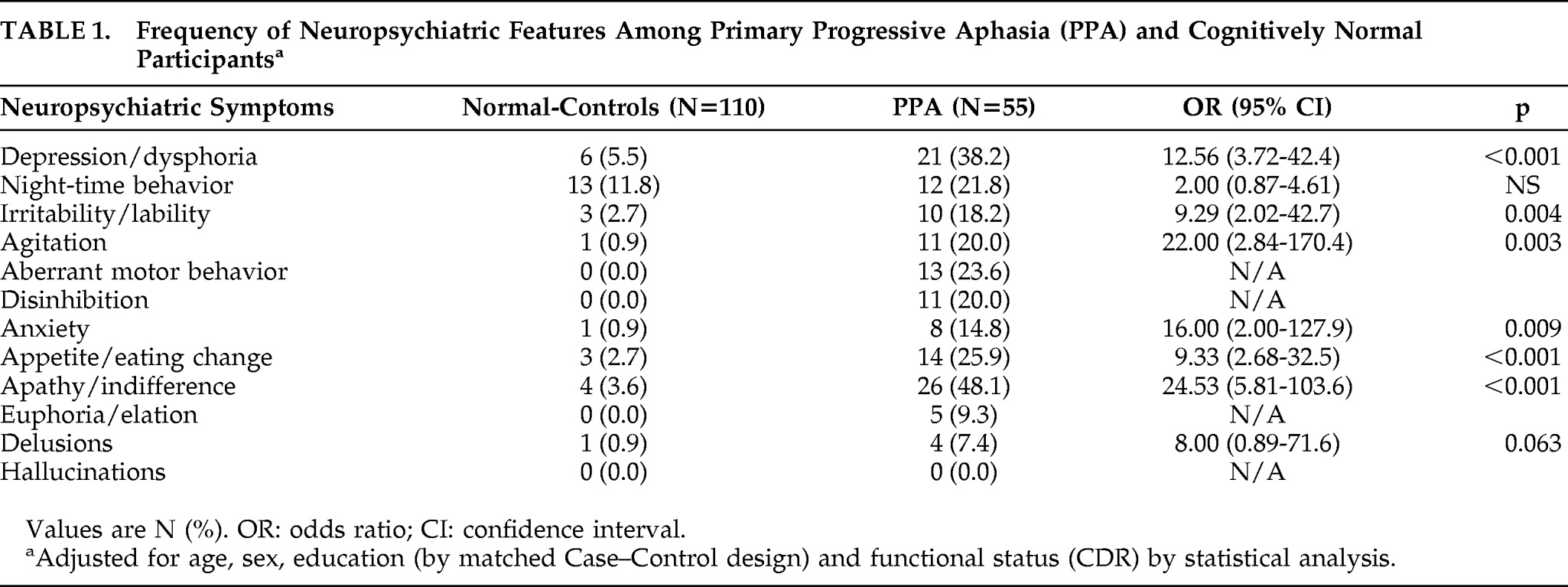
The frequency distribution of NPS is shown in
Table 1. The comparison of NPS between PPA Cases and Controls was made after adjusting for age, sex, education, and CDR. The median (range) NPI score for PPA group was 2.0 (0.0–9.0), whereas it was 0 (0–5) for the cognitively normal group. The most distinguishing features between subjects with PPA and Controls were in apathy (p<0.001), agitation (p=0.003), anxiety (p=0.009), depression (p<0.001), appetite change (p<0.001), and irritability (p=0.004). Delusions, euphoria, and hallucinations were rare or absent in both the PPA and cognitively normal groups. Aberrant motor behavior and disinhibition were present in only PPA subjects. Night-time behaviors were not found to be significantly associated with PPA. Further details of the neuropsychiatric data comparing subjects with PPA and cognitively normal persons are shown in
Table 1.
DISCUSSION
Here, we report a Case–Control study that compares the frequency of neuropsychiatric features of 55 cases of PPA with 110 cognitively normal persons. The neuropsychiatric comparisons were made after adjusting for potential confounders. The matched Case–Control design ensured that the PPA Cases were not different from Controls in age, sex, and education. Functional status as measured by CDR was adjusted by analysis. We observed that PPA is associated with depression, apathy, agitation, anxiety, appetite change, and irritability. Symptoms such as hallucinations and delusions were virtually absent in PPA. Night-time behavior was not significantly associated with PPA. Our findings are consistent with what was observed by Banks and Weintraub, who were perhaps the first to systematically examine neuropsychiatric symptoms in PPA.
13 They used the NPI-Q to measure neuropsychiatric symptoms in PPA (N=42) and then compared symptoms with those of bvFTD (N=28). They observed that the two groups differed in both quantity and quality of NPS wherein they observed depression, anxiety, and irritability in PPA, whereas apathy, disinhibition, and aberrant motor behaviors were more common in the bvFTD group. The investigators further divided the PPA group into early-stage (<5 years) and late-stage (≥5 years), based on the duration of the illness. They noted that patients with early-stage PPA tended to have mood symptoms, whereas late-stage PPA tended to show more symptoms of disinhibition and night-time behaviors; we also did not observe hallucinations, delusions, and night-time behaviors to be significant problems in PPA.
Our findings are also consistent with depressive symptoms observed by other investigators.
1,12,19 In 1982, when Mesulam first reported on PPA, four of the six PPA patients showed signs of reactive depression, sadness, and distress after the onset of PPA.
1As discussed above, NPS, particularly nonpsychotic symptoms, are associated with PPA. This is a cross-sectional association and does not mean that it is a cause-and-effect association. Also, our study was not designed to determine neuropsychiatric symptoms that are unique to PPA. A future prospective study that compares NPS with PPA and other neurodegenerative disorders (e.g., PPA versus Alzheimer's disease or FTD) is needed to identify characteristic neuropsychiatric features of PPA. Similarly, our study was not designed to examine the mechanism of disease; nevertheless, it is important to speculate on potential mechanisms that link PPA with NPS. Since PPA is a predominantly left-hemisphere disease, we will briefly examine theories relevant to NPS and hemispheric dysfunction. The reader is referred elsewhere for a comprehensive and authoritative review of the interaction of emotional disorders and neurological diseases.
23 Heilman and colleagues trace the work in the area of emotional experience and mood to Goldstein, who, as early as 1948, commented on the association between anxious depression and left-hemisphere lesions.
23 Studies involving patients with cerebral infarcts indicate that left-sided lesions, particularly those more anteriorly located, tend to be associated with depression.
20 This has been replicated in other stroke/depression studies as well as in left-sided brain-injury studies.
21,22 Heilman suggests that, in the normal state, the left side of the brain imparts a positive bias to emotional experience; therefore, in pathological states, the normal bias to hedonia is lost, and the patient's emotional state becomes negative (dysphoric). In contrast, the right hemisphere imparts a negative bias to emotional experience, so that stroke of the right hemisphere is associated with euphoria, since the normal negative bias is lost in a pathological state.
24 Heilman's theoretical model may be relevant to depression and PPA. Other theories have also been proposed to explain the laterality of emotional experience and behavior, including feedback and central theories that do not appear to be applicable to NPS and PPA; therefore, the reader is referred elsewhere for detailed discussion of the pathophysiology of emotional disorders related to hemispheric dysfunction.
23In summary, this study indicates that NPS, particularly nonpsychotic symptoms, are associated with PPA. To our knowledge, this may be one of the few studies that has systematically examined these associations. However, our findings need to be replicated by a prospective cohort study. Also, future studies need to be conducted to examine potential mechanisms linking NPS with PPA. At this point in time, we speculate that neuropsychiatric symptoms are either an emotional reaction to language impairment or a noncognitive manifestation of the neurodegenerative process driving both the PPA and NPS.
Acknowledgments
The authors are grateful for the statistical assistance of Ms. Teresa Christianson.
The preparation of this manuscript was supported by K01 MH068351, Harold Amos Medical Faculty Development Program (RWJ foundation), U01 AG06786, P50 AG016574, Robert H. and Clarice Smith and Abigail Van Buren Alzheimer's Disease Research Program.