Depression is a common mental disorder that is widely distributed in the population, with lifetime prevalence of 16.2%.
1 Despite the presence of a wide armamentarium of available treatment options, over 30% of patients still fail to attain remission.
2 In recent years, interests in developing other treatment modalities, such as vagal nerve stimulation, repetitive transcranial magnetic stimulation (rTMS), magnetic seizure therapy, and deep brain stimulation, have gained momentum.
3Barker et al.
4 developed rTMS, which allowed noninvasive stimulation of the cerebral cortex. Research in this field has increased, and recent metaanalyses have shown that rTMS over the left dorsolateral prefrontal cortex (DLPFC) is superior to sham stimulation
5–7 and is comparable to medication in its efficacy.
8 It is also emerging as a less-invasive alternative treatment to electroconvulsive therapy (ECT).
7,9 Furthermore, it appears as a promising approach to treatment-resistant depression.
10Depression may respond to either high-frequency (>1 Hz) rTMS applied to the left prefrontal cortex
11,12 or low-frequency (<1 Hz) rTMS over the right prefrontal cortex.
13 Most researchers argued that depression involves a hypoactive left DLPFC,
14–16 whereas others have suggested that dysfunction of the right DLPFC may be responsible for the disorder.
17 On the basis of these hypotheses, high-frequency, or excitatory, rTMS was applied to the left DLPFC, and low-frequency, or inhibitory, rTMS was applied to the right DLPFC. With the recognition of low-frequency rTMS as a possible alternative to high-frequency rTMS, Padberg et al.
18 compared the effects of high-frequency, low-frequency, and sham rTMS to the left prefrontal cortex on pharmacotherapy-resistant major depression. They found a significant decrease in the mean Hamilton Rating Scale for Depression (Ham-D) score only in the low-frequency (0.3-Hz) group after 5 days of daily treatment. Klein et al.
13 and Kauffmann et al.
19 reported decrease in depression scores in sham-controlled studies of medication-resistant major depression.
Despite the positive findings of both right- and left-sided rTMS, the degree of clinical effect still falls short of clinical application, and reasons cited have been many.
10 For example, despite consistent and large treatment effects, the average reduction in depression scores was only 37% (SD: 29%), and few patients met criteria for response.
10 In this context, there has been a quest for methods for enhancing response rates to rTMS treatment and the degree of response experienced by individual patients. A number of potential ways have been suggested, including optimizing pulse number and intensity, increasing the treatment duration, selecting appropriate patients,
20,21 bilateral stimulation,
22,23 and alternative treatment sites, such as the parietal cortex and cerebellum.
24 Neuro-navigational methods for localizing treatment sites have met with little success.
25 Another novel technique to improve response to rTMS is through priming. Priming stimulation has been explored in neurophysiological experiments that involve brief pretreatment with 5 Hz–6 Hz of stimulation preceding 1-Hz stimulation, to produce a decrease in synaptic efficacy.
26 Subthreshold 6-Hz rTMS or 6-Hz frequency-modulated (4–8 Hz) priming stimulation was found to reinforce the depression of motor responses induced by suprathreshold 1-Hz rTMS applied subsequently over the motor cortex.
27A PubMed search of priming and rTMS in depression resulted in one study by Fitzgerald et al.,
28 wherein the effect of 6-Hz priming followed by 1-Hz rTMS to the right DLPFC was compared with sham priming in 60 treatment-resistant patients with depression. A significant reduction in the Montgomery-Asberg Depression Rating Scale (MADRS) score was seen in the group receiving active priming rTMS. It has been shown that rTMS increases cortical inhibition in subjects with reduced baseline inhibition, which is consistent with the concept of homeostatic plasticity.
29 Stimulation using short bursts of three low-intensity pulses delivered within theta range can produce a lasting and more powerful effect on human motor cortex.
30 Therefore, the current study investigated the role of frequency-modulated priming stimulation in theta range (4 Hz–8 Hz) in treatment of depression.
Our objective was to compare the efficacy of adjuvant frequency-modulated active priming rTMS with sham priming stimulation in patients with moderate-to-severe depression receiving low-frequency rTMS. We hypothesized that there would be no significant difference in the efficacy of adjuvant frequency-modulated active priming rTMS as compared with sham priming stimulation in patients with moderate-to-severe depression receiving low-frequency rTMS.
MATERIALS AND METHODS
Study Sample
This was a prospective, hospital-based, sham-controlled rTMS study conducted over a period of 9 months, from April to December 2008 at the Centre for Cognitive Neurosciences of Central Institute of Psychiatry, Ranchi, India. The study was approved by the ethical committee of our Institute.
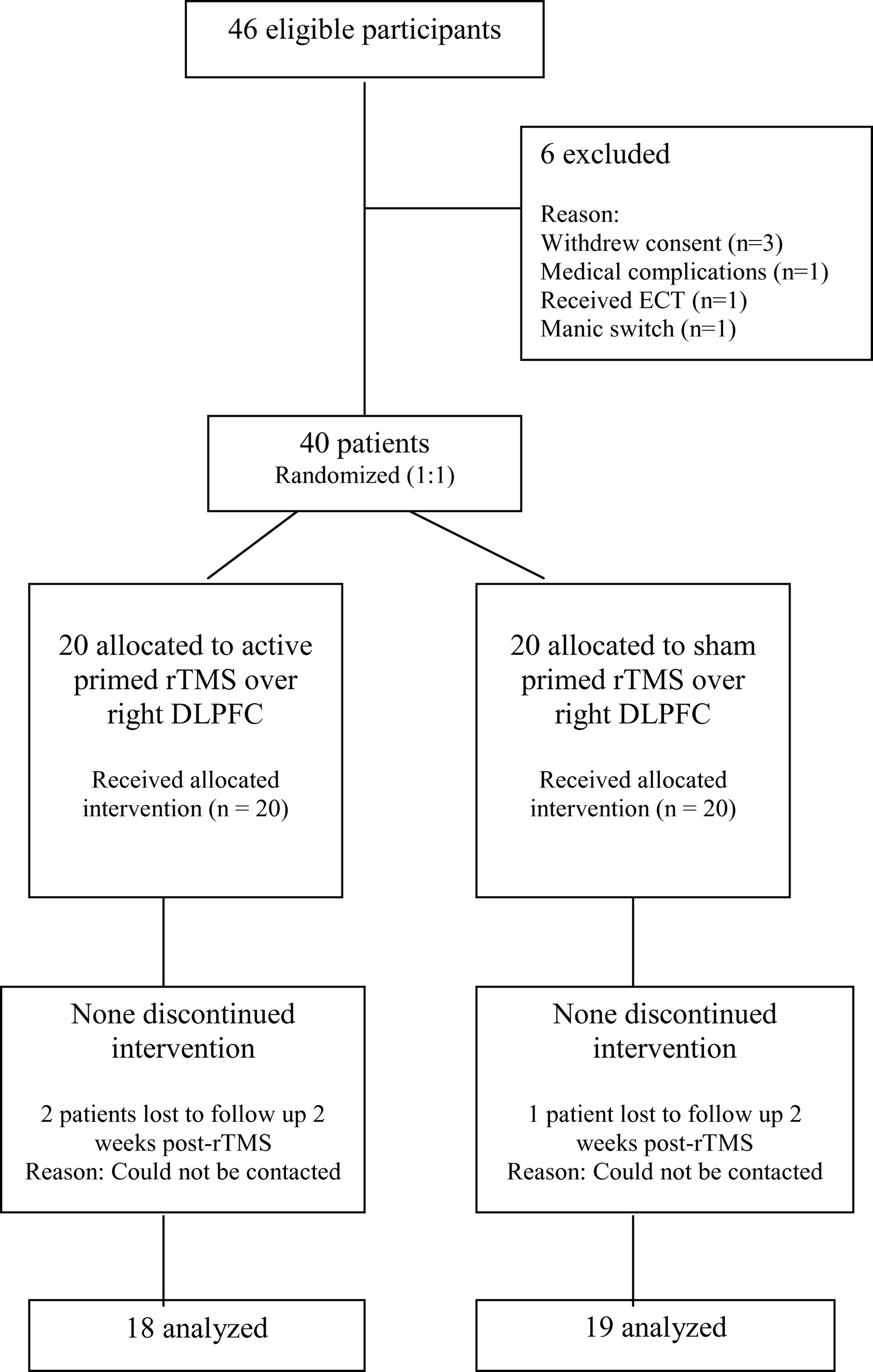
Figure 1 shows the CONSORT diagram of flow of participants through the trial. The sample consisted of 40 right-handed, normotensive patients of either sex, ages between 18 and 60 years, fulfilling the diagnosis of unipolar or bipolar, moderate-to-severe depression according to ICD–10 Diagnostic Criteria for Research,
31 and giving written informed consent. Patients with epilepsy, current neurological condition, comorbid psychiatric disorders, history of drug abuse, significant head injury, neurosurgical procedure, subjects with cardiac pacemakers or other metal parts in the body, and those who had received ECT in the past 6 months were excluded from the study. The selected 40 patients were alternatively assigned to receive either active priming rTMS (N=20) or sham priming stimulation (N=20), with the first patient receiving active treatment, the second receiving sham stimulation, and so on.
Tools
A semistructured pro-forma was used for recording sociodemographic and clinical details. To assess handedness, a 15-item Hindi version of the Handedness Preference Schedule
32 was used. A 21-item, clinician-administered Structured Interview Guide for the Hamilton Depression Scale (SIGH–D)
33 and 18-item Brief Psychiatric Rating Scale (BPRS)
34 were used to assess severity of depressive and psychotic symptoms, respectively. Treatment-emergent manic symptoms were assessed with the 11-item Young Mania Rating Scale (YMRS).
35 The Clinical Global Impression scale (CGI)
36 was used to assess overall illness severity and response to treatment.
Motor Threshold
The motor threshold (MT) for the left abductor pollicis brevis (APB) was determined using a Neuropack Sigma evoked-potential measuring system (Nihon Kohden, Japan) and a figure-eight shaped coil at 1-Hz frequency according to the Rossini-Rothwell algorithm.
37 According to this, MT was defined as the lowest intensity that produced 5 motor evoked potential (MEP) responses of at least 50 μV in 10 trials when the left APB is at rest. Mapping studies have found that the greatest responses for APB or first dorsal interosseous (FDI) stimulation are derived from coil (center) placement in a lateral-sagittal orientation at a point 2 cm. behind and 4 cm. to the left of the nasion-inion line.
38 Starting from this region, the stimulations were given at 1 Hz, and the coil was methodically moved across the right fronto-parietal region of the cranium, centered at the above-indicated point, until the motor cortex for the APB was located. Up to 10 single pulses were given at each level of intensity. Beginning at 50% intensity, it was increased by 5% and the procedure repeated until MT for APB was achieved. The right DLPFC rTMS stimulation site was determined by measuring 5 cm. anterior and in a parasagittal line from the point of maximum stimulation of the contralateral APB muscle.
39 The site was then marked for reference with an indelible skin-marker.
38Procedure for rTMS
Right 1-Hz stimulation in both arms of the trial (active priming and sham priming) were provided, using a Magstim Rapid device (The Magstim Company Limited, Whitland, UK) in one continuous, 15-minute train, at 110% of the resting MT, delivering 900 stimulations per session, using an air-cooled, figure-eight shaped coil. The priming stimulation that preceded the 1-Hz train was provided at 4 Hz–8 Hz (frequency-modulated in theta range). Twenty trains of 20 stimulations (total of 400 pulses), each at 90% of the resting MT, were applied in sequences of 7 trains, at 4 Hz, 7 trains at 6 Hz, and 6 trains at 8 Hz. For the sham group, the same stimulation condition was used, but with the sham coil.
The SIGH–D, BPRS, YMRS, and CGI were administered at baseline, after the 5th rTMS, after the 10th rTMS, and 2 weeks post-rTMS by the first author, AN. The rater was not blind to the treatment assignment, although the patients were blind to their treatment status. The stimulation sessions were performed as adjuncts to the ongoing medications.
Statistical Analysis
The results obtained were analyzed by the software program, Statistical Package for Social Sciences (SPSS Version 10.0 for Windows; SPSS, Inc.; Chicago, IL, U.S.). Sociodemographic, clinical, and pharmacological profiles were compared by independent-sample t-tests for continuous variables and chi-square test for categorical variables. In three patients, the final rating could not be completed (two in the active, one in the sham group); therefore, they were excluded from the final analysis. The effect of priming rTMS was determined with two-way, repeated-measures ANOVA, with Group (active and sham) and Time (baseline, after 5th rTMS, after 10th rTMS, and 2 weeks post-rTMS) as factors. Greenhouse-Geisser correction was applied because the sphericity assumption was violated. Post-hoc independent t-tests were carried out to find the differences in scores at all time-points. Effect size (η2) was calculated to quantify the strength of the treatment procedure in bringing about changes in various clinico-psychopathological variables. Covariate analysis was done to identify factors that explain the differences in baseline SIGH–D scores. Pearson's correlation coefficient was calculated between SIGH–D scores and sociodemographic and clinical variables. Stepwise linear-regression analysis was carried out using SIGH–D scores as the outcome variable to identify the predictors of response. In this study, a level of significance α of<0.05 (two-tailed) was taken to consider a result statistically significant.
RESULTS
Safety
There were no major adverse events during or after rTMS. Two patients (10%) in the active group and one patient (5%) in the sham group complained of headache lasting a few hours after rTMS, which resolved after they were given analgesics; headache did not recur in later sessions of rTMS. Other side effects reported were scalp tenderness in four patients (20%) in the active and three (15%) in the sham group; one patient (5%) in the sham group reported discomfort caused by twitching of the temporalis muscle during stimulation. None of patients switched to mania.
Sample Characteristics
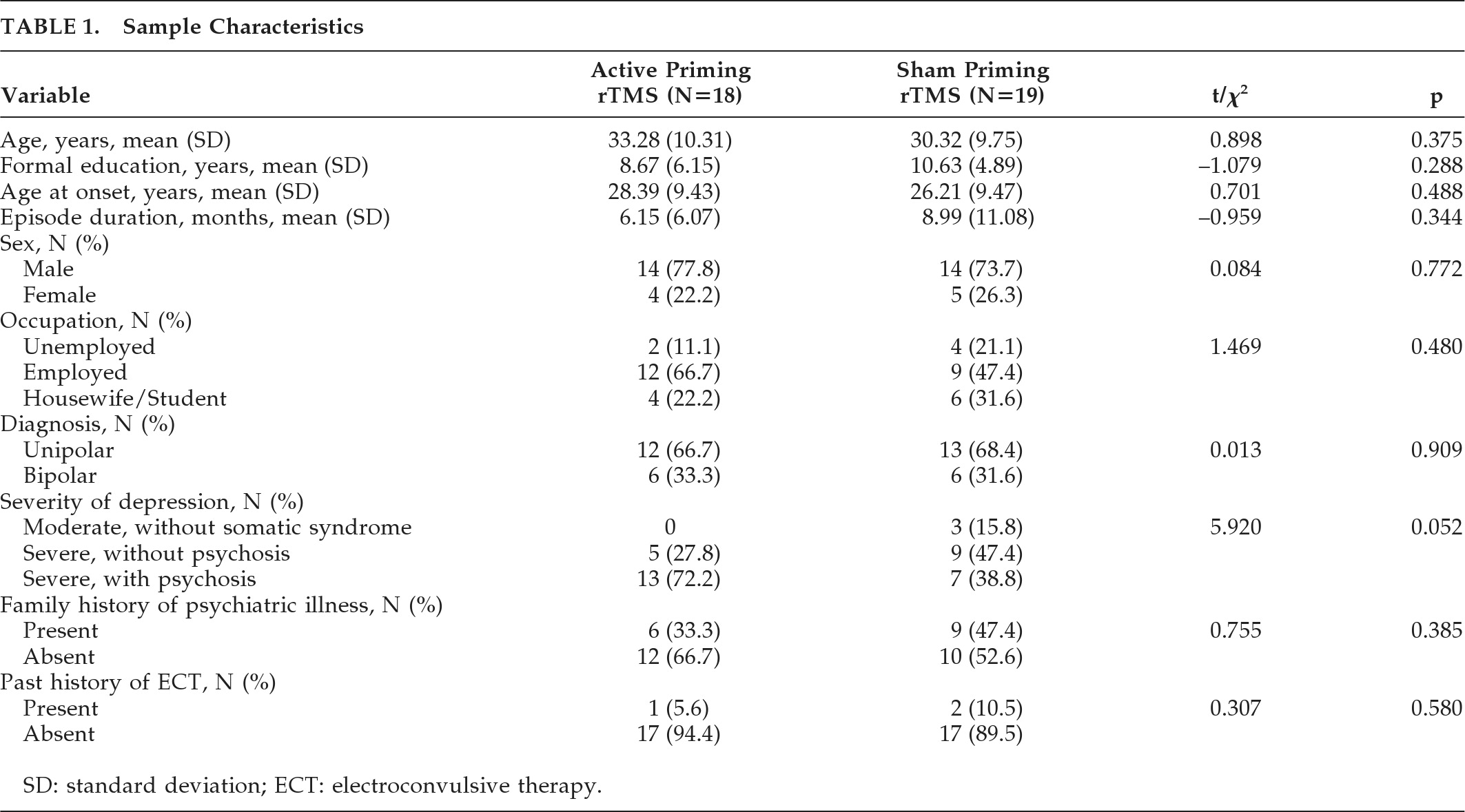
Sociodemographic and clinical characteristics of the sample have been summarized in
Table 1. Mean age of patients in the active priming rTMS group was 32.25 (SD: 10.38) years, and 30.50 (SD: 9.52) years in the sham priming rTMS group; there was no difference between them. In both the groups, 14 patients (70%) had unipolar, and 6 (30%) had bipolar depression.
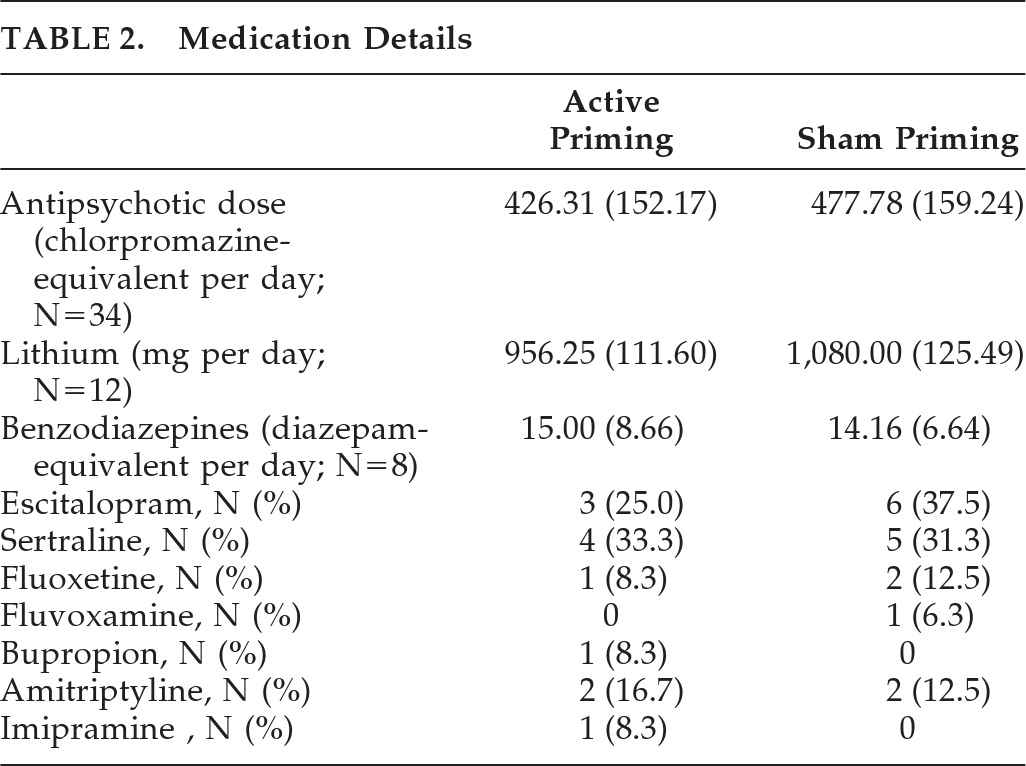
Table 2 shows medication details of both groups. The active group (N=19) received a mean dose of 426.31 (SD: 152.17) mg of chlorpromazine-equivalent of antipsychotics, versus 477.78 (SD: 159.24) mg/day in the sham group (N=18); this difference was not significant (
t[35] = –1.01; NS). Mean doses of lithium were 956.25 (SD: 111.60) mg/day in the active group (N=8), versus 1,080 (SD: 125.49) mg/day in the sham group (N=5), and a trend toward difference was seen (
t[11] = –1.858; p=0.09). In the active group (N=3), mean benzodiazepines (calculated in diazepam-equivalent) dose was 15.00 (SD: 8.66) mg/day, versus 14.16 (SD: 6.64) mg per day in the sham group; there was no difference between the groups. One patient in the sham group had received carbamazepine at 600 mg/day as a mood-stabilizer. There were nine patients on sertraline, nine on escitalopram, three on fluoxetine, four on amitriptyline, and one patient each on bupropion and imipramine. Mean MT was 53 (SD: 2.99) and 52.75 (SD: 3.43) in active priming and sham priming rTMS, respectively, with no significant difference between them.
Primary Outcome
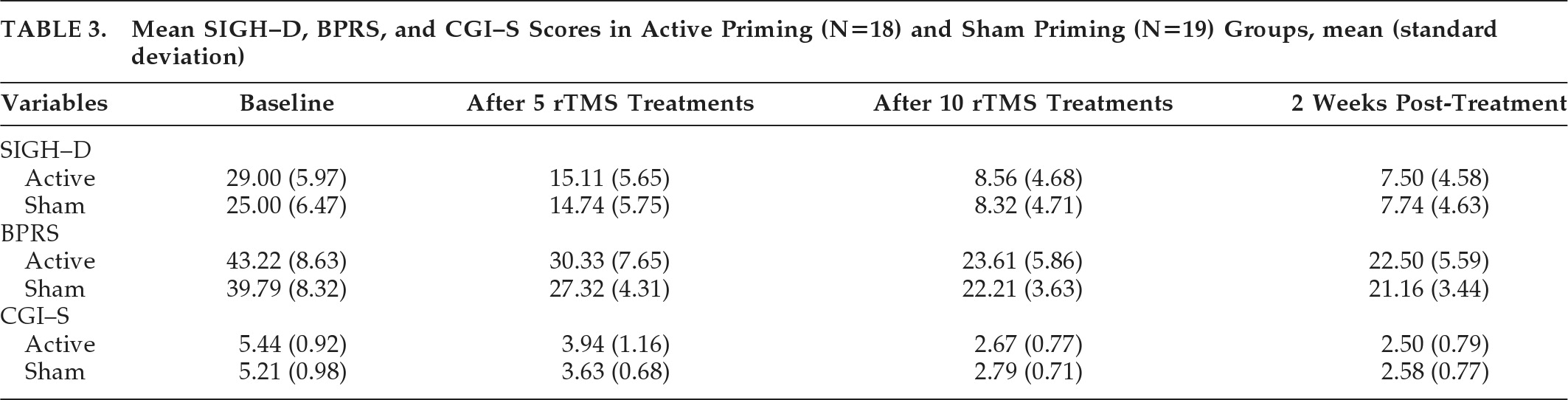
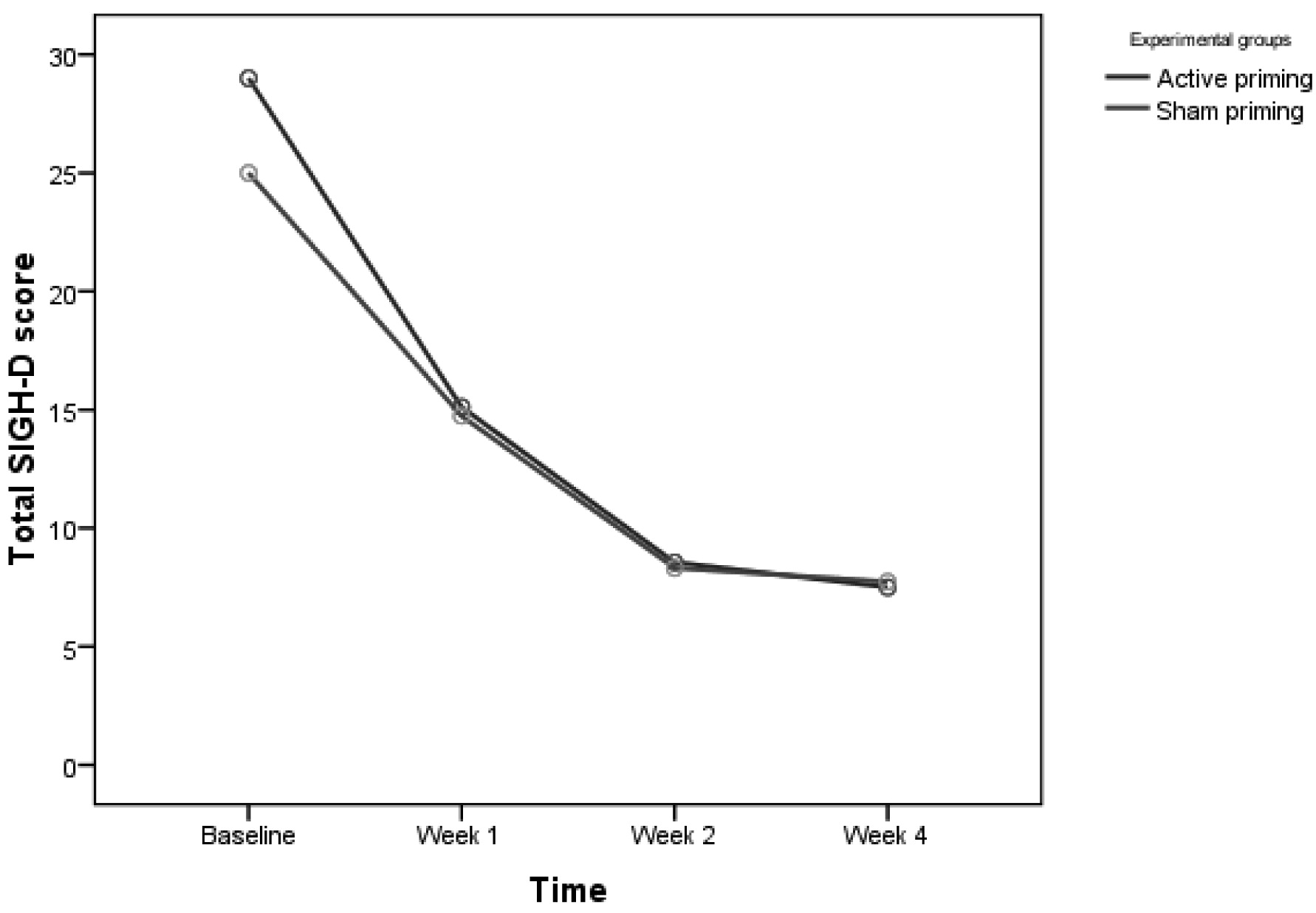
Table 3 shows mean SIGH–D, BPRS, and CGI–S scores in the active and sham groups. For SIGH–D scores, two-way, repeated-measures ANOVA showed significant main effect of Time (
F[2.12,74.23]=296.39; p<0.001, Greenhouse-Geisser–corrected; effect size: η
2=0.894), but no effect of Group (
F[1,35]=0.52; p=0.474). There was a significant Group x Time interaction in SIGH–D scores (
F[2.12,74.23]=3.53; p=0.032, Greenhouse-Geisser–corrected; effect size: η
2=0.092) showing significant improvement in the active group over time. Post-hoc independent
t-test between the two groups showed a trend toward significance in baseline SIGH–D scores, with a higher score in the active priming group (
t[35]=1.951; p=0.059), whereas, at other time-points, there was no difference (
Figure 2). Covariate analysis for age, age at onset of illness, and episode duration were not significant for baseline SIGH–D scores between active and sham priming groups. After controlling for antipsychotic dose (chlorpromazine-equivalent per day), there was a trend toward greater reduction in SIGH–D scores in the active priming group over time (
F[2.14,66.39]=2.51; p=0.086, Greenhouse-Geisser–corrected; effect size: η
2=0.075).
Controlling for BPRS scores at baseline, the reduction in SIGH–D scores between the two groups over time was no longer significant (F[2.42,82.23]=2.01; p=0.131, Greenhouse-Geisser–corrected). On further analysis of SIGH–D, we found that 12 patients (66.7%) receiving active priming rTMS were in remission (score of ≤8 on SIGH–D) at 2 weeks post-rTMS, versus 12 (63.2%) in the sham priming group. The number-needed-to-treat (NNT) analysis showed that 29 patients would need to be treated to benefit one person receiving active priming rTMS as compared with sham priming rTMS.
Secondary Outcome
For BPRS scores, two-way repeated-measures ANOVA showed significant main effect of Time (F[1.70,59.48]=138.25; p<0.001, Greenhouse-Geisser–corrected; effect size: η2=0.798), but no effect of Group (F[1,35]=2.19; NS) or Group x Time interaction (F[1.70,59.48]=0.50; p=0.580, Greenhouse-Geisser–corrected). For CGI–S scores, two-way repeated-measures ANOVA showed a significant main effect of Time (F[2.08,72.65]=155.34; p<0.001, Greenhouse-Geisser–corrected; effect size: η2=0.816), but no effect of Group (F[1,35]=0.154; NS) or Group x Time interaction (F[2.08,72.65]=1.14; p=0.326, Greenhouse-Geisser–corrected).
Correlation of SIGH–D Scores with Sociodemographic and Clinical Profile
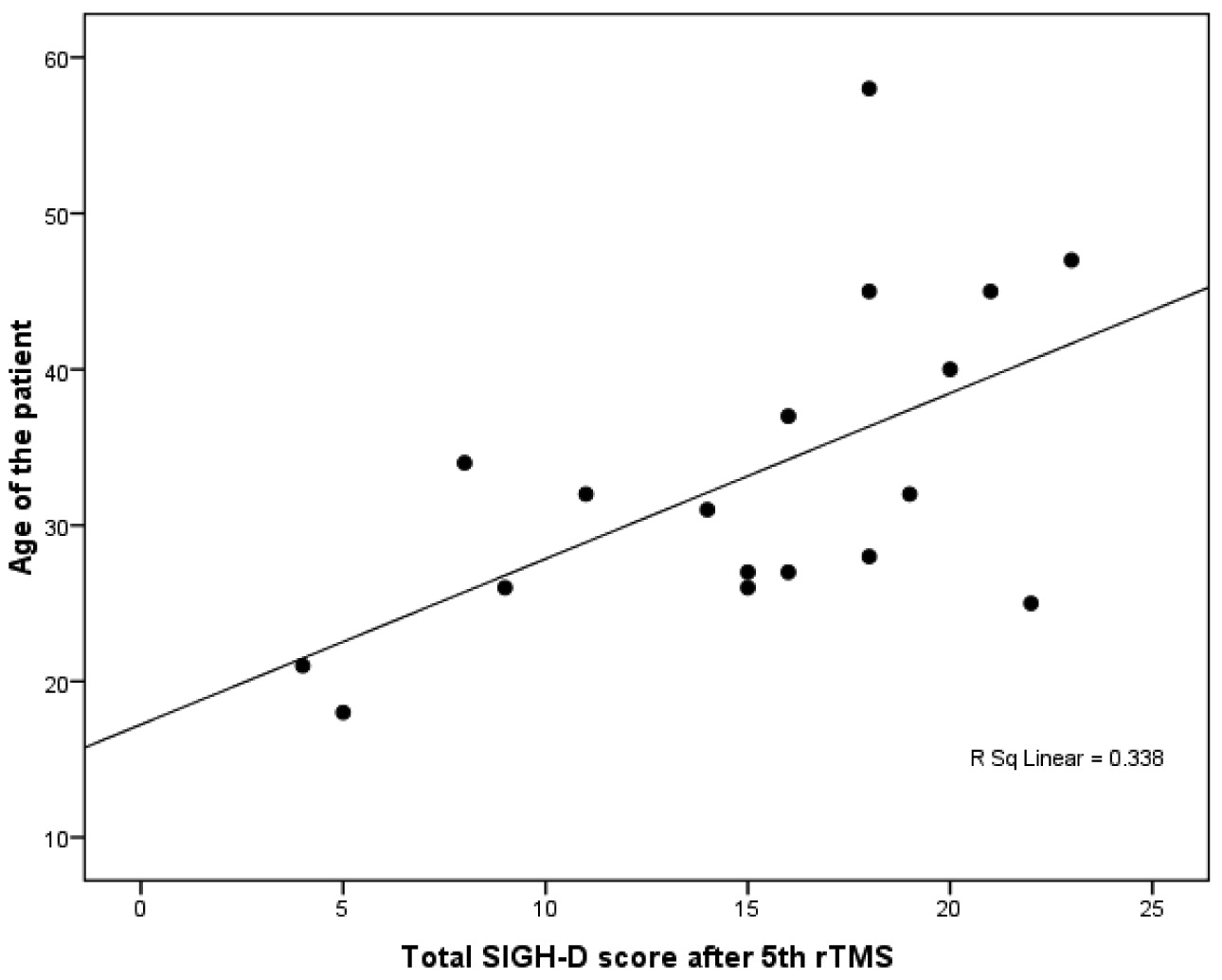
In the patients receiving priming stimulation, SIGH–D score after the 5th rTMS showed significant positive correlation with age (r=0.581; p<0.05) and age at onset of illness (r=0.641; p<0.05;
Figure 3), whereas SIGH–D score at baseline, after the 10th rTMS and 2 weeks post-rTMS did not show any significant correlation. In the sham priming group, we found no significant correlation between SIGH–D scores with sociodemographic or clinical parameters.
Predictors of Response
Stepwise linear-regression analysis was carried out using the SIGH–D score after the 5th and 10th rTMS, as well as 2 weeks post-rTMS, as outcome variables and age of patient, age at illness onset, and episode duration as predictors in both active and sham priming group. Only age at onset significantly predicted SIGH–D scores after the 5th rTMS (F [change]=11.19; p=0.004; β=0.384; R2=0.411) in the active priming group.
DISCUSSION
In our study, the effect of treatment measured over time with treatment as between-group factor, showed statistically significant improvement in the group receiving priming stimulation, as compared with the sham group, as shown by the change in SIGH–D scores (p<0.05), albeit with a small effect size.
40 This finding was in accordance with the findings of Fitzgerald et al.
28 and further strengthens the hypothesis that priming pre-stimulation enhances the effect of low-frequency rTMS through the modification of synaptic activity; that is, potentiation of a long-term depression (LTD) mechanism. Iyer et al.
27 had found no difference between 6Hz or frequency-modulated priming, and they had suggested that any frequency in the 4 Hz–8 Hz range (theta range) might be as effective as any fixed-frequency priming paradigm. Our study had used frequency-modulated priming in the 4 Hz–8 Hz range, and our findings are comparable to those of the Fitzgerald et al.
28 study, and in agreement with the findings of Iyer et al.
27In our study, both active and sham groups showed significant improvement over time, with large effect sizes.
40 This could be due to both active and sham groups having received a full course of active, low-frequency rTMS of the right PFC, which has been shown to be effective in depression,
13,41,42 along with standard pharmacotherapy. In our sample, a high proportion of patients had psychotic depression, as our Institute is a tertiary referral center that specializes in treatment of severely mentally ill patients. The rTMS group receiving priming stimulation showed no significant effect on BPRS scores over time when compared with the sham group (p>0.05). A possible reason could be that rTMS is not effective in psychotic depression and is comparable to ECT in depression without psychosis.
20,21,38 Furthermore, rTMS has been reported to worsen psychotic symptoms in depression.
43The mean age of patients was 32.25 (SD: 10.38) years in the active group and 30.50 (SD 9.52) years in the sham group, which was lower than that in the study by Fitzgerald et al.,
28 in which the mean ages were 45.7 (SD: 10.8) and 44.8 (SD: 11.4) years in the active and sham group, respectively. The baseline SIGH–D scores were 28.75 (SD: 5.88) and 24.60 (SD: 6.54) in the active and sham group, respectively, which was comparable to other studies of right-sided rTMS.
41,44 Both groups were comparable in terms of sociodemographic and clinical variables.
In our study, age at onset of illness positively predicted depression scores after rTMS. This suggests that the lower the age at onset of illness, the lower the depression scores, and vice versa. Our finding is in agreement with another study,
45 finding the antidepressant effect of TMS therapy to be better in younger and less treatment-resistant patients. One possible explanation could be that, with increasing age, there is an increase in scalp-to-prefrontal cortex distance and lesser neuroplastic changes. Hence, the magnetic field fails to induce electrical activity in the underlying cortical tissue, and, therefore, localized neuronal depolarization is prevented, resulting in lower response to rTMS in the older age-group.
44,46,47Limitations of our study included the lack of double-blinding, which could result in rater bias. The success of blinding in the patients was also not checked systematically. There was less representation of female patients, which limits generalization. The assignment of sample to active and sham treatment was done by purposive sampling, which does not involve random selection. Hence, it not a true randomization method and may potentially introduce bias. It is possible that the baseline differences in SIGH–D scores could be the result of a suboptimal randomization procedure. The dorsolateral prefrontal cortex of patients was located using the “5-cm rule,”
12 which does not take into consideration the shape and size of the participant's head. This may result in some variations in the exact site of stimulation in the prefrontal cortex. Also, priming in non-theta frequency range was not studied, which could shed light on the differential efficacy of priming in the theta-frequency range. Also, there is a possibility that the small effect of treatment is because of placebo response, which is seen when only mild-to-moderate depression patients are included.
48 This seems unlikely in our study because all patients had a moderate-to-severe level of depression.
On the basis of our findings, it can be concluded that pre-stimulation with frequency-modulated priming stimulation in the theta range has a greater antidepressant effect than low-frequency stimulation alone. Further double-blind, sham-controlled studies are required to optimize data on priming stimulation in depression.