Limbic encephalitis, a prototypic autoimmune neuropsychiatric disorder, is recognized by neurologists and psychiatrists by its subacute onset and rapid progression of cognitive, mood, and behavioral symptoms. However, autoimmune neurological disorders may present primarily as disorders of affect and thought and are underrecognized in psychiatric practice.
1–7 For example, Dalmau et al. reported that 77% of 100 patients with autoimmune encephalitis targeting
N-methyl D-aspartate (NMDA) receptors initially presented to a psychiatrist with symptoms of anxiety, agitation, bizarre behavior, delusional or paranoid thoughts, and visual or auditory hallucinations.
1 The entity of NMDA receptor autoimmunity was not recognized until seizures, movement disorders, hypoventilation, or coma ensued. A recent study illustrated that 6.5% of a cohort of patients presenting with a first episode of psychosis had autoantibody findings supporting an autoimmune diagnosis.
8Autoantibodies that serve as markers of autoimmune neuronal hyperexcitability disorders target macromolecular complexes containing the α-dendrotoxin-sensitive subset of the Kv1 family of voltage-gated potassium channels (VGKC). These channels are critical for regulating neuronal excitability.
9 In addition to VGKCs, these complexes contain cell adhesion molecules, membrane-associated guanylate kinases, cytoskeletal scaffold, disintegrin, and metalloproteinase 22 (ADAM22), and a soluble binding partner of ADAM22, leucine-rich, glioma-inactivated 1 (Lgi1) protein, which was first recognized as an epilepsy-linked protein.
10,11 Disorders initially encountered as neurological accompaniments of VGKC complex autoimmunity were Isaac syndrome (neuromuscular hyperexcitability), Morvan syndrome (accompanied by insomnia, autonomic dysfunction, and cognitive disturbance), and limbic encephalitis.
12–14 Recent evidence suggests that the majority of autoantibodies identified in clinical radioimmunoassays target neuronal proteins that co-immunoprecipitate with detergent-solubilized VGKCs. The principal antigens identified to-date include Lgi1, in patients with limbic encephalitis, and contactin-associated protein 2 (Caspr2), in some patients with acquired neuromuscular hyperexcitability, steroid-responsive peripheral neuropathies, dysautonomias, and encephalopathies.
15,16 VGKC complex antibodies are encountered in both an idiopathic and paraneoplastic context in adult and pediatric patients.
17 A variety of cancers has been reported in 47% of seropositive adult patients.
14 Caspr2-reactive IgG has been reported in VGKC-seropositive neurological patients with thymoma.
16Recent reports that some cases of VGKC autoimmunity can mimic Creutzfeldt-Jakob disease and frontotemporal dementia suggest the existence of a broader neuropsychiatric phenotype for presentation than identified to-date.
18,19 Patients with VGKC autoimmunity may present with psychiatric symptoms, including panic attacks
20and obsessive-compulsive behaviors.
21 Here, we report the frequency and spectrum of neuropsychiatric presentations of VGKC complex autoimmunity in consecutive seropositive patients seen at Mayo Clinic over a 14-month period.
METHOD
Patients
Study subjects were patients identified as seropositive for VGKC complex autoantibody among 6,814 Mayo Clinic patients (Rochester, MN; Scottsdale, AZ; and Jacksonville, FL) tested serologically (with IRB approval) in the period from June 1, 2008 to July 31, 2009.
Clinical Evaluation
We reviewed medical records of all seropositive patients and recorded demographic information, medical history, examination findings, neuropsychiatric symptoms, and laboratory data (imaging, serologic, neuropsychometric). Patients were classified as having florid neuropsychiatric decompensation (i.e., subacute multi-domain neuropsychiatric dysfunction associated with marked functional impairment) or milder neuropsychiatric presentations. We also documented neurological and cancer diagnoses.
Serological Evaluation
All patient sera were tested by a standardized, clinically validated, radioimmuno-precipitation assay using VGKC complex proteins solubilized in digitonin from porcine cerebral cortical membranes and radioligated with
125I-labeled α-dendrotoxin (which binds with high affinity to a Kv1 subset of channels). Results are expressed as nanomoles of VGKC complex bound per liter of serum.
13 No patient's serum bound to
125I-α-dendrotoxin alone.
22 All sera were additionally evaluated by standardized immunofluorescence criteria for IgG neural autoantibodies (antineuronal nuclear antibody [ANNA]-1, 2, 3; amphiphysin antibody, Purkinje cell [PCA]-1, 2 and Tr antibodies; collapsin response-mediator protein [CRMP]-5 IgG and antiglial/neuronal nuclear antibody [AGNA-1]); by radioimmuno-precipitation assays for neuronal voltage-gated cation channel antibodies (neuronal calcium channels [P/Q-type and N-type]), muscle and neuronal ganglionic (α3) nicotinic acetylcholine receptor (AChR), and glutamic acid decarboxylase 65-isofrom (GAD65); and by recombinant Western blot for collapsin response-mediator protein (CRMP)-5-IgG.
23,24 Thyroid autoantibody testing results were obtained from the patients' medical records.
Statistical Analysis
We divided patients into three groups according to VGKC complex antibody values: Low (0.03–0.09 nmol/liter), Medium (0.10–0.99 nmol/liter), and High (>1.00 nmol/liter).
25 We compared the frequency of symptoms between Low and Medium/High antibody-value groups by use of Fisher's exact test (two-tailed). A two-sided p <0.05 was considered significant. To determine whether variables other than the VGKC complex antibody value might contribute to a patient's neuropsychiatric presentation or florid neuropsychiatric decompensation, we performed logistic-regression analysis including VGKC complex antibody values; seropositivity for other neural, muscle, or thyroid antibodies; and psychiatric predisposition (past psychiatric history), and we compared immunotherapy outcomes (Wilcoxon rank-sum test).
RESULTS
Of 6,814 patients evaluated for paraneoplastic autoantibodies in a 14-month period, 152 patients (2.2%) were seropositive for VGKC complex antibodies (median value: 0.15 nmol/liter; range: 0.03–14.5 nmol/liter; normal range: 0.00–0.02 nmol/liter). Of those, 50% were current or former smokers; 55% were women; 91% were white; the median age was 59 years (range: 2–87).
Patients With Neuropsychiatric Symptoms
Sixty-seven patients (44%) presented with one or more neuropsychiatric symptoms. Those 67 patients are the subjects of this report. Twenty-one (31%) had a documented history of psychiatric illness, versus 21 of 85 (25%) with a non-neuropsychiatric presentation (p=0.369). Eighteen patients (27%) had a formal psychiatric evaluation, 5 by mental health care providers before evaluation at Mayo Clinic, and 13 during the course of Mayo Clinic evaluation. Seventeen patients (25%) had an autoimmune history: thyroid disease, 9; pernicious anemia, 3; rheumatoid arthritis, 2; ulcerative colitis, 2; and type 1 diabetes mellitus, 1. The initial diagnosis in two patients was a primary psychiatric disorder.
Patient 91:
Insomnia and mood change developed subacutely in a 45-year-old man with no past psychiatric history. Treatment with hypnotics and sertraline did not improve symptoms. After development of anxiety and muscle twitching, he had a psychiatric admission, and a diagnosis of depression was made. Neuropsychometric testing demonstrated impairment of verbal fluency, cognitive speed, and executive functioning. EEG revealed epileptiform discharges. Abnormal autoantibody findings included VGKC complex Ab (0.06 nmol/liter) and thyroid peroxidase Ab (165.4 IU/ml). Reevaluation after plasma exchange documented improvement of cognition and anxiety.
Patient 102:
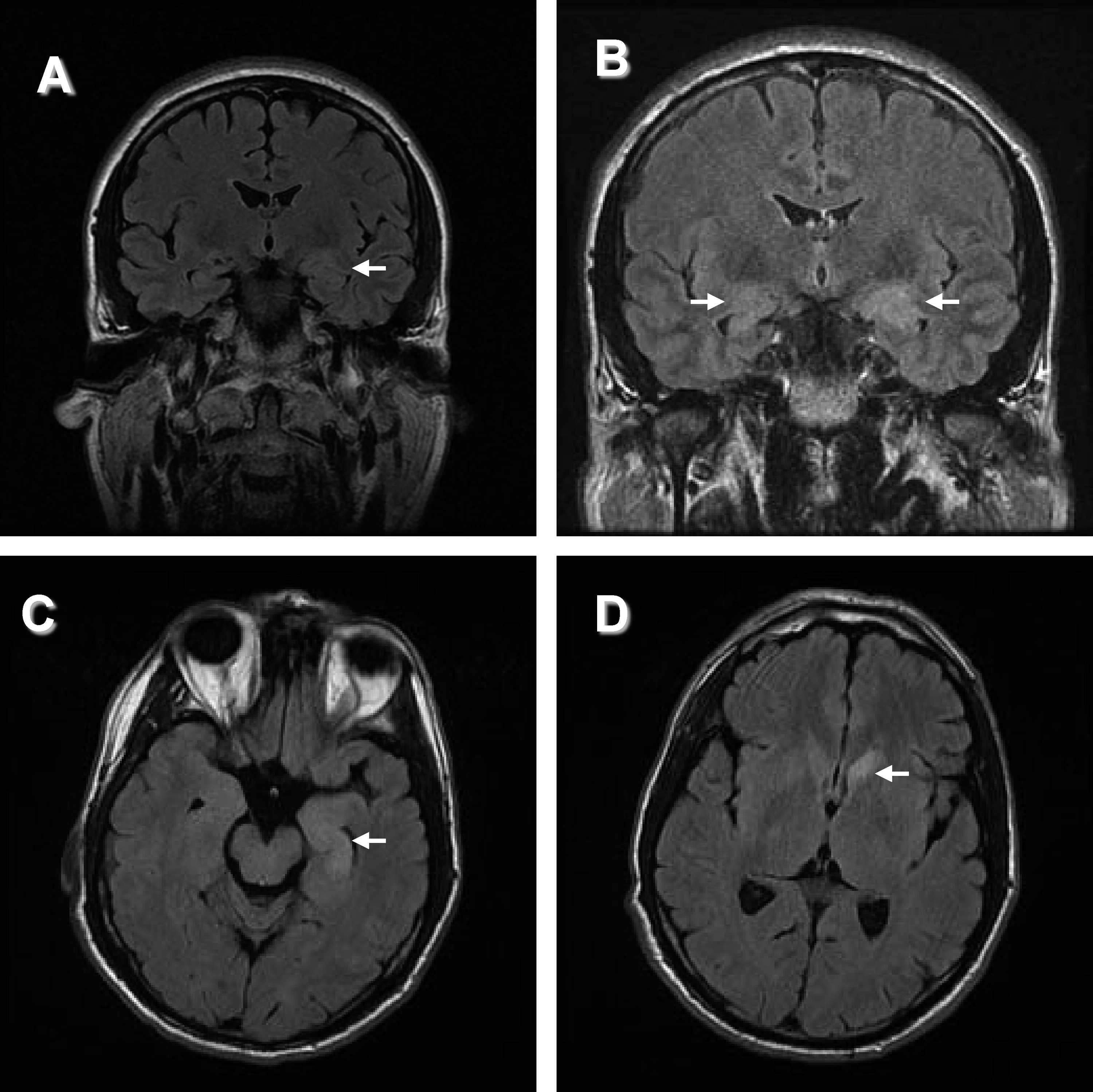
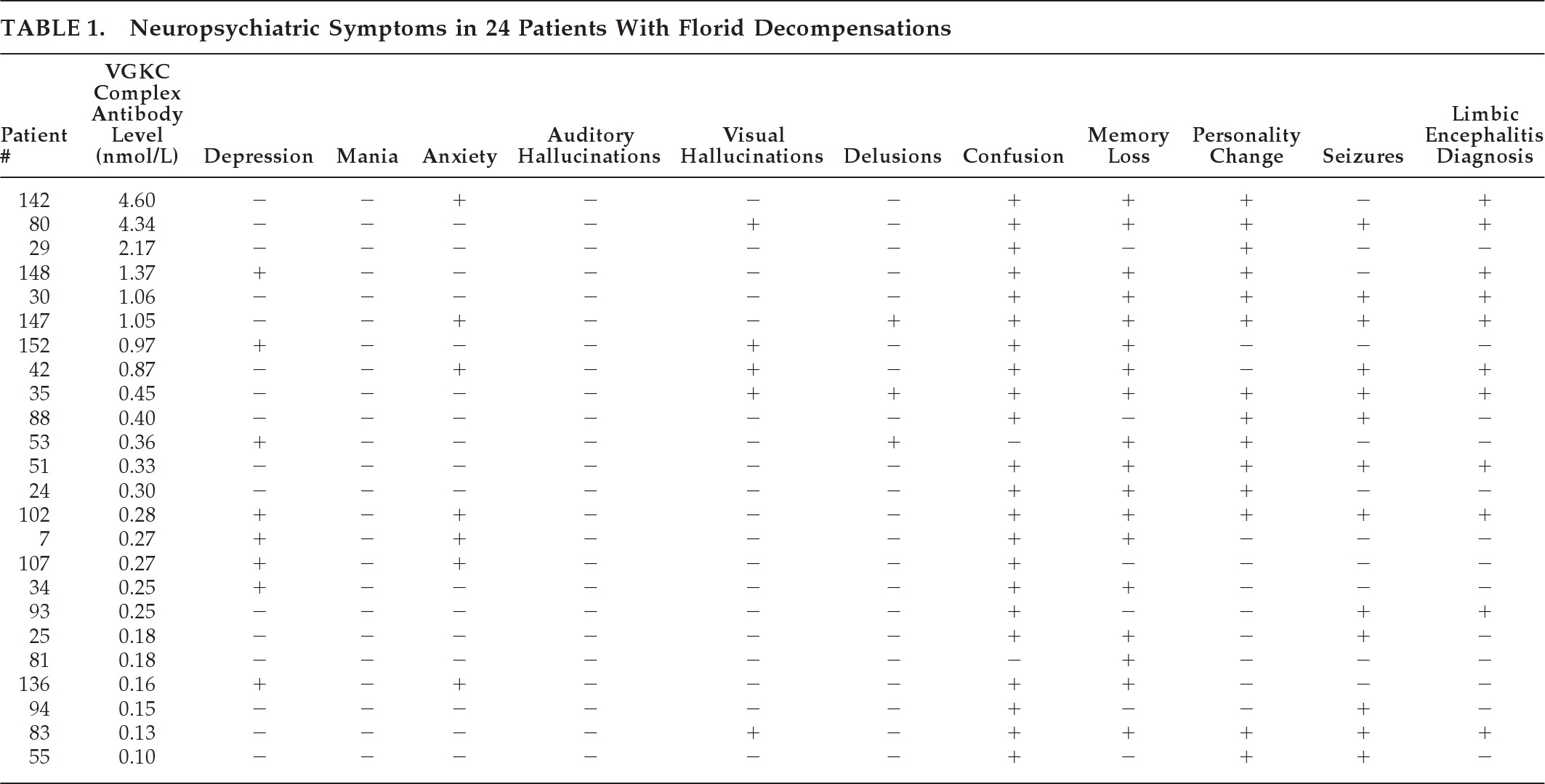
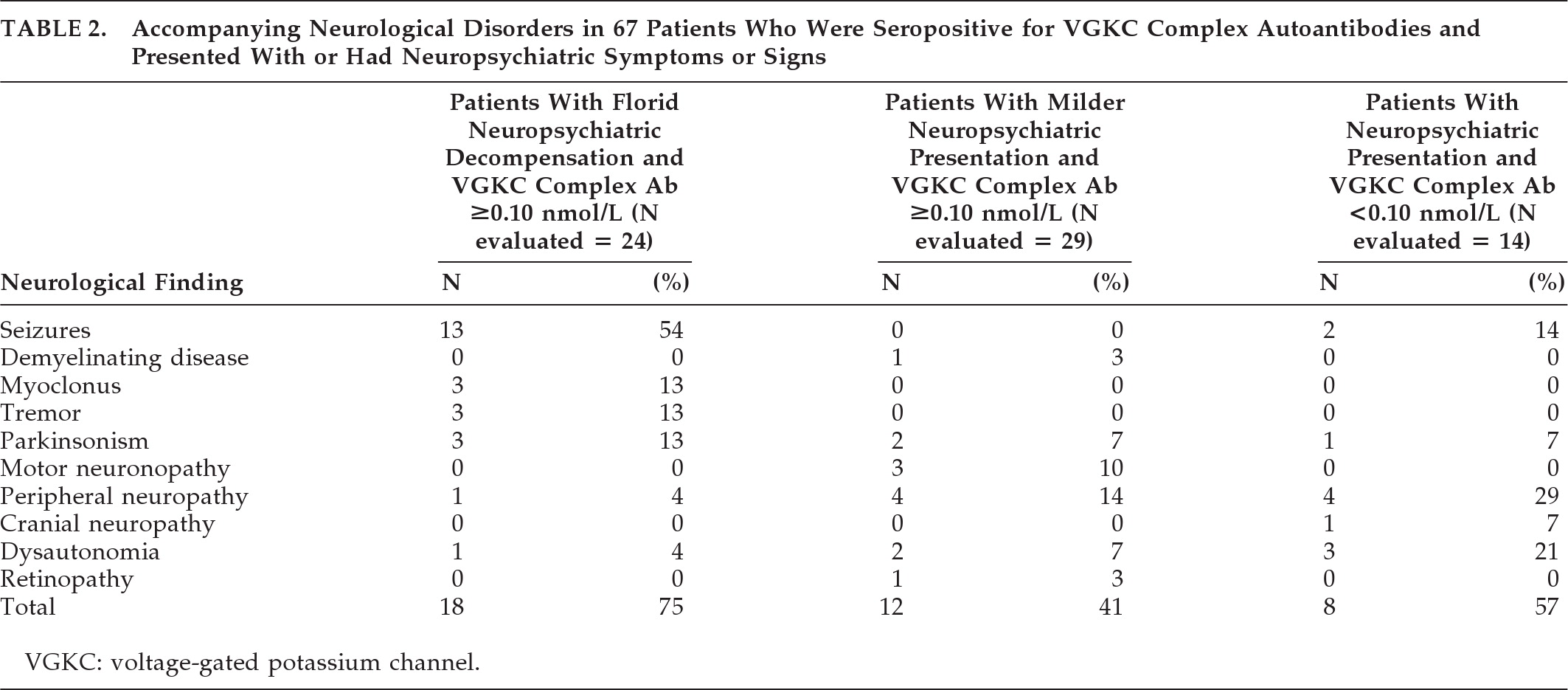
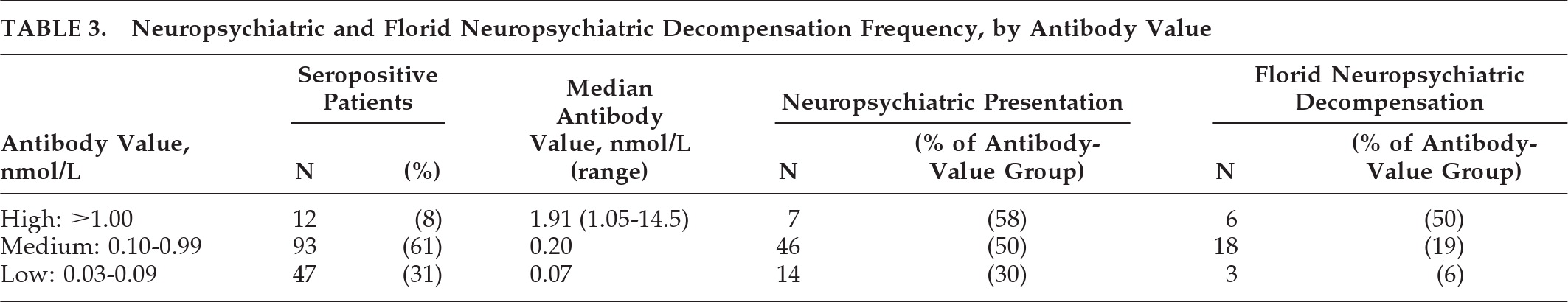
“Panic attacks” developed subacutely in a 53-year-old man with no past psychiatric history. He then developed episodes of fear, tachycardia, piloerection, chills, and amnesia. An MRI of the head (
Figure 1[A]) was reported as normal. Treatment with clonazepam did not improve the symptoms. Depression was diagnosed, but treatment with escitalopram was unsuccessful. Six months into his illness, a generalized seizure was witnessed; video EEG revealed left-temporal epileptiform discharges. MRI now clearly demonstrated bilateral hippocampal abnormalities. Levetiracetam treatment did not improve symptoms. Substitution with carbamazepine decreased the frequency of episodes. VGKC complex Ab was 0.28 nmol/liter. After subsequent initiation of immunotherapy and continued carbamazepine therapy, the seizures resolved. Upon re-evaluation by neuropsychometric testing, there was objective improvement of the amnesia.
Patients With Florid Neuropsychiatric Decompensation and VGKC Complex Ab Values ≥0.10 nmol/Liter
A total of 24 of the 67 patients presenting with neuropsychiatric symptoms had florid neuropsychiatric decompensation at onset (36%;
Table 1). The median autoantibody value among these patients was 0.32 nmol/liter (range: 0.10–4.60 nmol/liter).
Clinical Characteristics
Neuropsychiatric symptoms among the 24 patients with florid neuropsychiatric decompensation (
Table 1) included confusion, 22 (92%); memory impairment, 18 (75%); personality change, 14 (58%); depression, 8 (33%); anxiety, 7 (29%); visual hallucinations, 5 (21%); spells, 4 (17%); and delusions, 3 (13%). Sleep disorders (hypersomnolence, insomnia, or dream enactment) were reported in 8 (33%). Neurological signs were documented in 18 (75%;
Table 2). Electroencephalography (EEG) was performed in 22 of 24 (92%), and was abnormal in 16 (73%). A seizure disorder was diagnosed in 13 of 24 (54%). No patient who presented with a florid neuropsychiatric decompensation had affective symptoms alone.
Thirteen of 24 patients were not diagnosed with limbic encephalitis; their symptoms were confusion, 11; memory loss, 8; depression, 6; personality change, 5; seizures, 4; anxiety, 3; visual hallucinations, 1; delusions, 1. Just one patient had abnormalities on MRI suggestive of an autoimmune diagnosis (with widespread T2 signal changes atypical of limbic encephalitis). VGKC complex antibody values were not significantly different for this subgroup.
MRI Findings
MRI brain imaging was done in 22 of 24 patients (92%), and was normal in 4 (18%). Eleven had hippocampal atrophy or increased T
2 or FLAIR signal of the mesial temporal lobes (50%;
Figure 1), and one had widespread bihemispheric abnormalities. Other findings, not specific for an autoimmune diagnosis, included: leukoaraiosis, 7 (32%); mild-to-moderate generalized atrophy, 4 (18%); prominent perivascular spaces, 2 (9%); meningioma, 1 (5%); arachnoid cyst, 1 (5%); and chronic lacunar infarcts, 1 (5%).
Treatment and Outcomes
Eleven patients were diagnosed with classical limbic encephalitis (46%). Treatment information was available for 19 (79%) of 24 patients. Median duration of follow-up was 15 months (range: 5–27 months).
Immunotherapy
Fifteen of 19 patients (79%) were treated with immunotherapy. Treatments included: methylprednisolone, 15 (79%); mycophenolate mofetil, 8 (42%); pooled human immune globulin (IVIg), 7 (37%); plasma exchange (PLEX), 2 (11%); azathioprine, 3 (16%); methotrexate, 1 (5%); and rituximab, 1 (5%). Median time from illness onset to immunotherapy initiation was 8 months (range:1 month–60 months). Time to immunotherapy initiation could not be determined for one patient. Ten of 15 patients improved (67%). The delay from symptom onset to initiation of treatment was significantly shorter in patients for whom improvement was reported (median delay: 7 months; range: 2 months–11 months) than in those for whom no improvement was reported (median delay: 30 months; range: 17 months–60 months; p=0.0124). Ten patients (53%) required long-term immunosuppression because improvements observed after an initial course of immunotherapy were followed by objective (e.g., formal neuropsychometric testing, brief mental status testing, or EEG) or subjective clinical decline. Two patients (11%) were treated with more than one form of immunotherapy before symptoms remitted, but did not require long-term immunosuppression. Despite multiple forms of immunotherapy, two patients 11%) had no clinical improvement, and one (5%) died.
Patients With Milder Neuropsychiatric Manifestations and VGKC complex Ab values ≥0.10 nmol/Liter
Twenty-nine of 67 patients (43%) presented with milder neuropsychiatric manifestations in addition to other symptoms, mostly neurological. The median VGKC complex antibody value among these patients was 0.16 nmol/liter, range (0.10–1.19 nmol/liter).
Clinical Characteristics
Psychiatric symptoms in these 29 patients included depression, 12 (41%); anxiety, 11 (38%); memory impairment, 6 (21%); confusion, 4 (14%); hallucinations, 1 (3%), delusions, 1 (3%); and spells, 1 (3%). Twelve patients presented with both neuropsychiatric symptoms and neurological findings (41%;
Table 2). Sleep disorders (hypersomnolence, insomnia, or dream enactment) were reported in 7 patients (24%). Other reported symptoms were fatigue, 5 (17%); gastrointestinal symptoms (nausea, altered bowel habit), 4 (14%); muscle cramping or twitching, 4 (14%); extremity tingling, 4 (14%); dizziness, 3 (10%); skin discomfort (burning, rash, or swelling), 2 (7%); globus, 1 (3%); and joint pain or stiffness, 1 (3%).
MRI Findings
Eleven of 29 patients with milder neuropsychiatric presentation underwent brain MRI (38%). None had the mesial temporal abnormalities typical of limbic encephalitis. Findings reported were not diagnosis-specific and included leukoaraiotic or vascular changes (7; 64%) and focal or generalized atrophy (4; 36%).
Treatment and Outcomes
Treatment information was available for 13 of 29 patients (45%). Median duration of follow-up was 9.5 months (range: 3 months–28 months). Three of these 13 patients (23%) were treated with immunotherapy (one or more of IVIg, methylprednisolone, prednisone, and azathioprine); neuropsychiatric symptoms did not improve in any patient treated with immunotherapy.
Neuropsychiatric Symptoms in Patients With Low-Positive VGKC Complex Ab Values (<0.10 nmol/liter)
Neuropsychiatric symptoms also were recorded in 14 patients who had low-positive antibody values (0.03–0.09 nmol/liter). Presentations were florid in 3 and mild in 11, and included confusion, 7 (50%); memory impairment, 7 (50%); depression, 6 (43%); anxiety, 6 (43%); personality change, 2 (15%); spells, 1 (7%); and hallucinations, 1 (7%). Neurological signs were documented in 8 patients (57%;
Table 2). Treatment information was available for three patients who received immunotherapy; one improved.
Coexisting Antibodies and Oncological Findings in Patients With Neuropsychiatric Symptoms
One or more additional autoantibodies were detected in 17 of the 67 patients with neuropsychiatric presentations (25%). The specificities of accompanying autoantibodies were, in descending frequency: thyroid peroxidase, 8; striational, 6; glutamic acid decarboxylase 65 isoform (GAD65), 4; ganglionic AChR, 1; muscle AChR, 1. Three patients had multiple coexisting antibodies.
Among 49 patients evaluated oncologically, 12 (24%) had a cancer history documented or were found to have a cancer subsequent to VGKC complex detection. New cancers (1 recurrent) were detected in 7 patients (14%): prostate adenocarcinoma, 1; thyroid papillary carcinoma, 1; colon adenocarcinoma, 1; recurrent bladder carcinoma, 1; melanoma, 1; squamous cell carcinoma, 1; and acute lymphocytic leukemia, 1. Two malignancies (colon adenocarcinoma and thyroid papillary carcinoma) were occult until revealed by whole-body PET-CT imaging. A coexisting paraneoplastic autoantibody (targeting ganglionic AChR) was found in one patient in whom cancer was found (colon carcinoma). VGKC complex antibody values among patients with cancer (median: 0.27 nmol/liter; range: 0.06–4.34 nmol/liter) were not significantly different from values among patients without evidence of cancer (median: 0.16 nmol/liter; range: 0.03–4.60).
The patient who had colon adenocarcinoma achieved complete remission from neuropsychiatric symptoms after partial colectomy and subsequent immunotherapy. Encephalopathy did not recur after gradual withdrawal of immunotherapy. The patient who had thyroid papillary carcinoma achieved complete resolution of encephalopathy within 10 days after cancer resection alone.
VGKC Complex Antibody Values Among Patients With and Without Neuropsychiatric Symptoms
Among all 152 patients who were VGKC complex antibody-seropositive, 12 had values ≥1.00 nmol; 93 had values between 0.10 and 0.99 nmol/liter, and 47 had values of 0.03–0.09 nmol/liter (
Table 3). Individuals with medium or high VGKC complex antibody values were significantly more likely to have a neuropsychiatric presentation (odds ratio [OR]: 2.40; 95% confidence interval [CI]: 1.15–5.00; p=0.022) or a florid neuropsychiatric decompensation (OR: 4.35; 95% CI: 1.28–15.25; p=0.020) than patients with low VGKC complex antibody values. No individual neuropsychiatric symptom occurred more commonly in the combined Medium and High antibody value group than in the Low antibody value group.
Among those with a neuropsychiatric presentation, the VGKC complex antibody value was the only clinically significant variable in the logistic model (OR: 2.40; 95% CI: 1.15–5.20; p=0.019). In contrast, among those with a florid neuropsychiatric decompensation, both the VGKC complex antibody value and the presence of any coexisting antibody were significant (OR: 3.71; 95% CI: 1.18–16.42; p=0.023 and OR: 2.67; 95% CI: 1.06–6.69; p=0.038, respectively).
Diagnoses documented among the 85 patients without neuropsychiatric presentations were the following: peripheral nervous system disorders, 41; no definitive neurological diagnosis, 17; autonomic neuropathies, 10; myelopathy, 4; movement disorder, 3; optic neuropathy, 3; myasthenia gravis, 2; stroke, 2; cancer only, 2; seizures, 1.
DISCUSSION
Our analysis of data for a series of 67 consecutive Mayo Clinic patients with VGKC complex autoimmunity documents heterogenous neuropsychiatric manifestations; 36% had florid presentations. The high frequency of VGKC complex antibody detection (2.2% of patients tested) and the high prevalence of psychiatric symptoms among these patients, suggest that autoimmune psychiatric disorders are underrecognized. The Mayo Clinic patients we have described represent just 27% of all patients in whom a VGKC complex autoantibody was detected in the study period. Among the Mayo patients, we identified two who were treated for primary psychiatric diagnoses initially, but were subsequently determined to have an autoimmune, immunotherapy-responsive disorder.
Patients with Medium or High antibody values were significantly more likely to present with neuropsychiatric manifestations and florid neuropsychiatric decompensation than patients with Low antibody values. Although laboratory false positives are eliminated by evaluating nonspecific binding to the α-dendrotoxin radioligand, antibody values <0.10 nmol/liter sometimes lack a neurological correlate. The presence of an interfering monoclonal or polyclonal gammopathy should be considered.
25 Patient 91 (antibody value 0.06 nmol/liter) had a clearly immunotherapy-responsive disorder. Coexisting neural and thyroid autoantibodies were more common among patients with florid neuropsychiatric decompensations; 41% had coexisting autoantibodies, versus 15% among patients who presented with milder neuropsychiatric manifestations. This finding suggests that in some patients (for example, Patient 91), an immune response targeting multiple onconeural autoantigens may result in more florid neuropsychiatric symptoms.
Published reports of psychiatric autoimmunity are scarce, but include cases of depression,
2,3 bipolar disorder,
4 panic attacks,
20 obsessive-compulsive symptoms,
21 and schizophreniform illnesses
5–8 in the setting of an autoimmune diagnosis, either organ-specific (usually thyroid) or non–organ-specific (usually systemic lupus erythematosus). Patients reported previously had poor responses to standard psychopharmaco-therapies alone but remitted with immunotherapies (usually corticosteroids). Other data supporting an autoimmune etiology for some psychiatric symptoms include a higher incidence of thyroid disease and type 1 diabetes among first-degree relatives of patients with psychosis than among control subjects.
26Consistent with previous reports,
12–14 the most common symptoms and signs in patients with florid neuropsychiatric decompensation and VGKC complex Ab values ≥0.10 nmol/liter were those commonly seen in limbic encephalitis or similar encephalopathic states (memory loss, confusion, and seizures) whereas affective symptoms, such as depression, were less common; 75% exhibited objective neurological signs. In contrast, patients with milder neuropsychiatric presentations often had common affective psychiatric symptoms, such as anxiety and depression, and nonspecific somatic complaints. These patients were also less likely to demonstrate objective neurological signs (41%), and none had MRI findings suspicious for an autoimmune disorder. None improved with immunotherapy, but only 10% were treated. These patients may represent a continuum of less well-recognized VGKC complex autoantibody-associated psychiatric disorders.
Also consistent with previous reports, diverse cancer types were detected and were generally anatomically limited.
14 The oncological associations reported for VGKC complex autoantibodies to-date are neither specific for anatomical site nor histological type, and the positive predictive value is lower than for classical paraneoplastic antibodies (such as ANNA-1). Furthermore, patients with cancer are not readily distinguishable from those without cancer with regard to neurological presentation and VGKC complex antibody value, and most do not have coexisting antibodies predictive of cancer. Since VGKC complex antibody testing was done exclusively in the context of physician requests for a “paraneoplastic serological evaluation,” and not in any other context, the possibility of bias toward the detection of cancer in these patients exists. In the setting of negative standard testing, combined PET-CT scanning further increases the cancer diagnostic yield by 20%.
23 Patients treated oncologically or with immunotherapy within the first 12 months after presentation with a VGKC complex antibody-associated encephalopathy fared significantly better than those who did not receive treatment within the first year (p=0.0124). This finding supports previous observations that prompt treatment of autoimmune neurological disorders permits better outcomes.
27 Long-term immunotherapy is usually required to maintain remission from neuropsychiatric symptoms.
28Autoimmune psychiatry represents a new and exciting field of study. Future studies should prospectively explore the clinical and immunological aspects of VGKC complex autoimmune neuropsychiatric disorders. Standardized measures to detect neuropsychiatric signs and symptoms could be applied to a prospective study. This information will promote better understanding of the pathophysiology of these disorders and their responsiveness to immunotherapy and other treatments. Undoubtedly, mental health providers encounter patients with autoimmune neuropsychiatric disorders in their current practice. Improved awareness will facilitate recognition and appropriate treatment.
Acknowledgments
Part of this work was presented at the Academy of Psychosomatic Medicine Annual Meeting, Marco Island, FL, November 13, 2010.
The authors acknowledge the staff of the Neuroimmunology Laboratory, in particular Greg Kraus, for excellent technical support.
Dr. Lennon and Mayo Clinic have a financial interest in the following intellectual property: NMO-IgG: A Marker Autoantibody of Neuromyelitis Optica“ A patent has issued for this technology, and it has been licensed to commercial entities. Dr. Lennon has received cumulative royalties of less than the federal threshold for significant financial interest from the licensing of these technologies.
Drs. Lennon and Pittock have a potential financial interest in the following technologies: Aquaporin-4 Autoantibody as a Cancer Marker” A non-provisional patent application has been filed by Mayo Clinic for this technology and it has been licensed by Mayo Clinic to a commercial entity. No royalties have accrued from this license.
“Aquaporin-4-Binding Autoantibodies in Patients with Neuromyelitis Optic Impair Glutamate Transport by Down-Regulating EAAT2.” A non-provisional patent application has been filed by Mayo Clinic for this technology.
Drs. Somers, Rundell, Lachance, Drubach, Trennery, Klein, Aston and McKeon report no disclosures.