Buried amid the seemingly never-ending advance of the U.S. Food and Drug Administration's (FDA) black-box warnings on the labels of most psychiatric drugs available today, there is increasing debate on the power of those warnings to change physicians' prescribing behavior.
Over the last 16 months, the FDA has imposed new black-box warnings on all antidepressants marketed in the United States and all newer-generation antipsychotics as well as many older antipsychotic drugs. Last month an FDA advisory committee, after little discussion or debate, recommended that the agency add a new black-box warning to the labels of all stimulants used to treat attention-deficit/hyperactivity disorder (Psychiatric News, March 3).
However, one issue that was addressed by that committee—the Drug Safety and Risk Management Advisory Committee— involved speculation regarding the impact of the black-box warning. Some members hoped the impact would be significant, akin to the large drop in the number of antidepressant prescriptions dispensed to children and adolescents that followed warnings about the potential risk of suicidality. Other committee members were more skeptical, saying that most physicians today do not read or follow the advice given in the warning sections of drug labels.
Now, a new study looking at black-box warnings and the extent to which physicians follow them indicates that 1 in 10 patients whose records were reviewed was written a prescription for a medication that carried a black-box warning. More importantly, however, 7 in 1,000 patients received a prescription for a drug that was contraindicated because of a potential adverse interaction between the new medication and another drug the patient was already taking, or because the new drug should not have been prescribed to a patient with that particular diagnosis.
The study was funded by grants from the Harvard Risk Management Foundation and Partners HealthCare Information Systems and appeared in the February 13 Annals of Internal Medicine.
Researchers led by Karen Lasser, M.D., M.P.H., an instructor in the Department of Medicine at Harvard Medical School, reviewed the electronic health records of patients receiving care from Partners Health Care in the Boston area to determine how frequently physicians prescribed a drug contraindicated by a black-box warning and how frequently that prescribing resulted in harm. Partners Health Care is a group of ambulatory practices including 40 hospital-based clinics, four community health centers, and seven community-based practices. All prescribing for these outpatient practices was done electronically.
At the time of the study, however, the electronic prescribing system provided physicians with only drug-allergy cross-checking and default dosing suggestions. The system did not inform prescribers about black-box (or any other) warnings, check for interactions between the drug prescribed and other medications the patient was on, check for interactions between the drug prescribed and laboratory monitoring necessitated, or check for contraindications for the drug prescribed due to incompatibility with a patient's diagnosis.
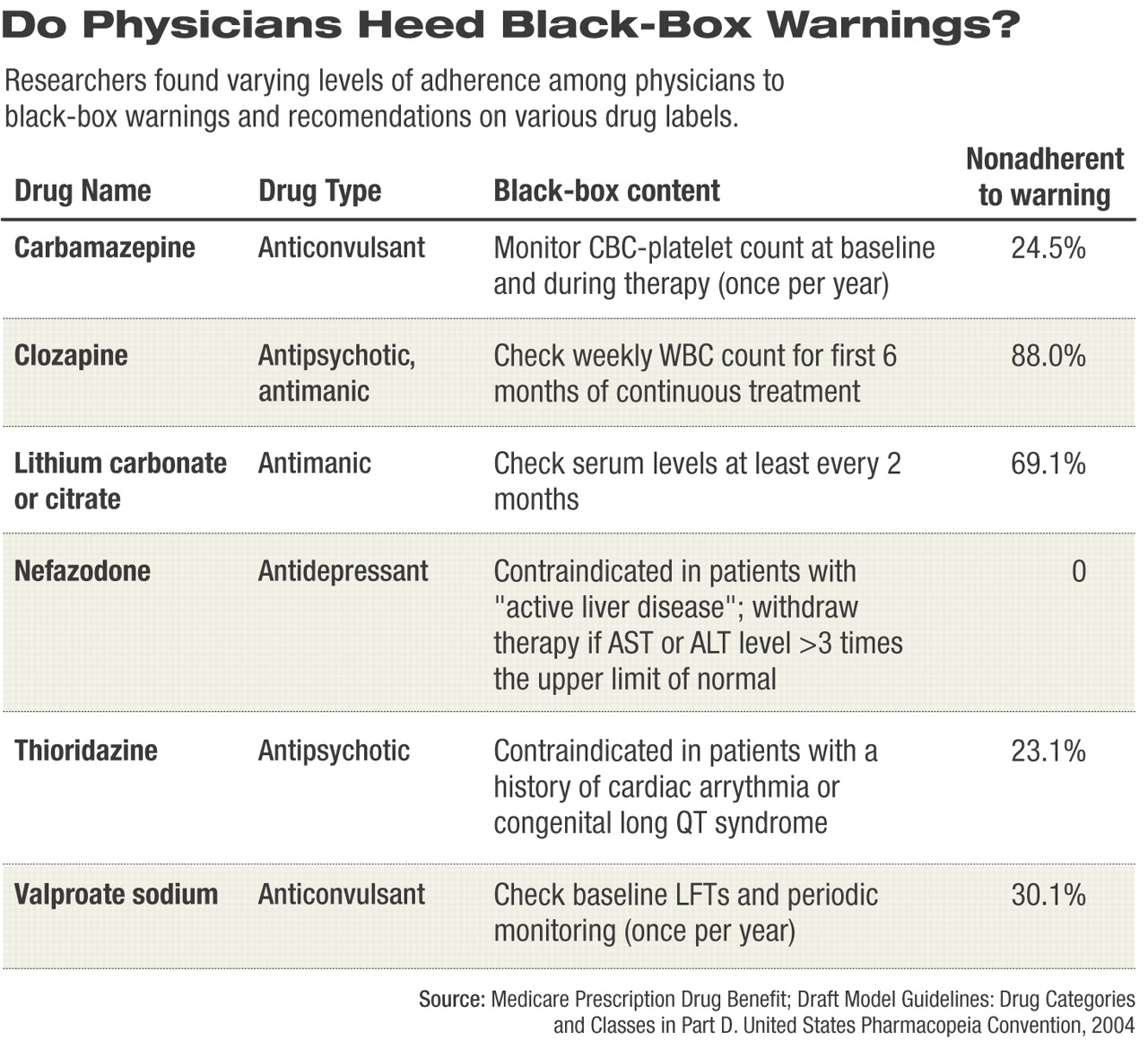
The researchers first identified all prescriptions written in 2002 for any medication carrying a black-box warning. They then looked at the records of individual patients who received prescriptions for drugs with black-box warnings to determine whether the warning should have precluded writing the prescription for that particular patient. The team designated prescriptions that should have been precluded as “black-box violations.”
Lasser and her colleagues identified 33,778 prescriptions written for drugs with black-box warnings, representing 10.4 percent of the total of 324,548 outpatient prescriptions written during 2002. However, of those 33,778 prescriptions, only 2,354 prescriptions (0.7 percent of all prescriptions) were written for outpatients who should not have received the particular medication prescribed.
The overwhelming majority of those prescriptions —1,744 (74 percent)—were written for medications with black-box warnings requiring some type of laboratory monitoring that was not adequately completed as recommended in the warning. A total of 401 prescriptions (17 percent) were written for patients who had a contraindicated disease, and 209 prescriptions were written for patients who were already taking a contraindicated drug that may have resulted in a serious drug-to-drug interaction.
The data compilation for the study was completed in early 2004, prior to the imposition of the black-box warnings on antidepressants (October 2004) and antipsychotics (April 2005). In addition, the researchers looked at all drug classes with black-box warnings in 2002, not just psychotropic medications. Overall, the researchers found that black-box warnings were most commonly not followed regarding prescriptions for several antineoplastic drugs and several anti-fungal medications.
However, prescriptions for several psychotropic medications were identified to which black-box warnings were frequently disregarded, mostly involving anticonvulsant/mood stabilizing medications. Nearly 25 percent of patients (129 of 526) identified by the study as taking carbamazepine had no documented evidence of monitoring of CBC and platelet counts at baseline and at least once each year during therapy. Nearly 70 percent of patients (266 of 385) taking lithium did not have serum lithium levels drawn every two months as advised in the drug's black-box warning.
Lastly the researchers determined what, if any, impact violating the black-box warnings had on patients. The researchers found four adverse drug events (ADEs) resulting from a violation of a black-box warning, three of which were rated as serious (requiring urgent medical intervention) and one less significant. There were 92 possible ADEs identified, 18 of which were rated as having a potential for a fatal or life-threatening ADE, 71 for a serious ADE, and the remainder for less significant ADEs. In addition, 154 medication errors were identified.
Overall, Lasser and her team found that patients who were female, were aged 75 or older, and took multiple medications (four or more) were at highest risk of a black-box violation compared with younger male, nonwhite patients, who took fewer medications.
Lasser and her colleagues said one solution for reducing black-box violations would be for the FDA to “make these warnings more specific so they are more readily understandable by providers.”
Arch Intern Med 2005 166 338