A substantial body of research has documented that up to 83 percent of the friends and family members of people diagnosed as having schizophrenia experience considerable financial, emotional, and practical burdens (
1 ). They report time lost from work, unreimbursed medical and other patient-related expenses, limited time for leisure and socializing, elevated symptoms of psychological distress, and feelings of stigmatization (
1,
2,
3,
4,
5,
6 ). In addition to its impact on caregiver quality of life, caregiving strain has been associated with other adverse effects, including poorer self-rated health, chronic medical conditions, or both (
7,
8,
9,
10,
11,
12 ); increased visits to a primary care physician (
8,
13,
14,
15 ); greater use of psychotropic drugs, such as tranquilizers and antidepressants (
16,
17,
18,
19 ); and increased risk of medical hospitalization (
8 ).
Despite these adverse effects of caregiving, efforts to identify contributing factors have mostly been limited to investigations of the impact of different types of patient symptoms, with mixed conclusions. Although one study found that higher levels of burden were associated with higher levels of negative but not positive symptoms (
19 ), others found that increased burden was associated with higher levels of both positive and negative symptoms (
1,
3,
20,
21,
22 ) or with positive symptoms alone (
23,
24 ).
Although theorists have identified multiple aspects of patient behavior that influence the caregiver's experience of burden (
25 ), few studies have examined the impact of aspects of the patient's life and functioning other than his or her symptom level on caregiver burden. To our knowledge, no study has evaluated the association between patient quality of life or neurocognitive functioning and family burden in schizophrenia beyond the impact of symptoms. To the extent that patients are optimally medicated with relatively stable symptom profiles, patients' quality of life, cognitive status, or both might carry greater prognostic weight than symptom measures. This difference might arise because patients who are more socially interactive outside the home, who have the cognitive capacity to be productively employed, and who have more satisfying interpersonal relationships with others make fewer demands on the caregiver or present fewer subjective concerns or worries to him or her. Conversely, patients whose symptoms are well controlled who do not develop a fuller life may concern caregivers because there is no ready solution at hand.
Similarly, no study has evaluated the associations between patient service use and presence of medication side effects with family burden in schizophrenia. The patient's use of and satisfaction with mental health services, as well as the presence of medication side effects, may be anticipated to influence the caregiver's evaluation of how effectively the patient's illness symptoms are being managed, and in turn, his or her perceived burden.
In the study reported here, we used baseline data from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study, a major multisite trial of antipsychotic pharmacotherapy funded by the National Institute of Mental Health to evaluate the relationships between patient symptoms, quality of life, service use, and caregiver burden, controlling for patient and caregiver sociodemographic variables (
26 ). Data were collected between 2001 and 2003. We anticipated that caregivers would experience less burden from patients who had lower symptom levels, a higher quality of life, superior cognitive functioning, fewer medication side effects, positive attitudes toward medication, more years in treatment, and less intensive current treatment.
Methods
Participants
Of the 1,460 patients enrolled in CATIE, 623 (43 percent) identified caregivers who agreed to be interviewed about their life situation and experiences with the patient and completed all measures. Caregivers were identified by the patient as the family member or friend most directly involved in his or her care. All patients and caregivers gave written informed consent to participate in protocols approved by local institutional review boards.
Measures
Patient measures. The diagnosis of schizophrenia was confirmed by the Structured Clinical Interview for Axis I and II DSM-IV Disorders (SCID) for all participants (
27 ). Symptoms of schizophrenia were assessed with the rater-administered Positive and Negative Syndrome Scale (PANSS), which yields a total average symptom score that is based on 31 items rated from 1 to 7 (with higher scores indicating more severe symptoms) as well as subscales reflecting positive and negative symptoms (
28 ). Depression was measured with the Calgary Depression Rating Scale (
29 ). Use of drugs and alcohol over the past three months was evaluated on a 5-point scale (1, abstinent; 5, dependent) with the clinician-rated Alcohol Use and Drug Use Scale (
30 ). Severity of antisocial behavior before age 15 was assessed with the sum of six items taken from the SCID, including violation of rules (for example, school truancy or expulsion), running away from home, destruction of property, aggression (initiation of physical fights), and trouble with the law (such as getting arrested).
Quality of life was evaluated by the Heinrichs-Carpenter Quality of Life Scale (
31 ), a 22-item rater-administered scale for assessing overall quality of life and functioning in four areas: intrapsychic foundations (such as motivation, curiosity, and empathy), interpersonal relations, instrumental role, and common objects and activities. Items were rated from 0 to 6, with higher scores reflecting better quality of life. In addition, questions from the Lehman Quality of Life Interview (
32 ) were used to evaluate the adequacy of the patient's finances over the past six months and the total number of hours of employment per week.
Service use variables represented use of mental health outpatient services and residential treatment in the past month, occurrence of an exacerbation of mental symptoms requiring psychiatric hospitalization or crisis stabilization during the past three months, use of any type of hospitalization for any reason in the past month (all coded as yes or no), total number of years in mental health treatment, and the patient's subjective response to medications, evaluated by the Drug Attitude Inventory (
33 ), a ten-item true-false scale, on which higher numbers indicate more positive views toward medication.
Medication side effects were assessed with the Barnes scale for akathisia (
34 ), the Abnormal Involuntary Movement Scale (AIMS) for tardive dyskinesia (
35 ), and the Simpson-Angus scale for extrapyramidal side effects (
36 ).
Neurocognitive functioning was measured by separate test scores, described in a previous publication (
37 ), which were converted to z scores and combined to construct five separate scales that were themselves averaged to form an overall neurocognitive functioning scale.
Caregiver characteristics and burden. Family burden was evaluated with an adapted version of the Family Experience Interview Schedule (FEIS) (
38 ), which evaluates patient problem behavior, activities of daily living, role functioning, disruption of household routine, caregiver contributions in time and money to the patient's general support and treatment, and the amount of practical and emotional support provided to the caregiver by the patient. Because the FEIS comprises scales and items selected conceptually on the basis of findings of prior studies of family burden (
38 ), an approach adopted by more recent studies as well (
39 ), we used factor analysis to reduce the FEIS to dimensions that were statistically as well as conceptually meaningful. The pool of 44 variables and scales derived from the FEIS were subjected to an exploratory principal-components factor analysis with orthogonal (varimax) rotation. Data were randomly divided into two approximately equal samples of 304 and 319 with the SPSS sample procedure (
40 ) for use in our exploratory and confirmatory analyses.
Exploratory analysis
Examination of the skree plot of eigenvalues indicated a four-factor solution, and we eliminated six items that failed to load on any factor. Factor 1, problem behavior (
Table 1 ), represented illness-related behaviors problematic to caregivers, such as violent outbursts, referential thinking, and excessive use of drugs and alcohol. Factor 2, resource demands and routine disruption, captured demands on the caregiver and household, such as financial costs, social limitations, and strained relationships. Items loading on factor 3, impairment in activities of daily living, related to patients' functional ability or activities of daily living, including meal preparation and hygiene. The fourth factor, perceived patient helpfulness, assessed the degree to which the patient helped the caregiver emotionally, financially, and practically, such as by participation in household chores.
Confirmatory analysis
Results of the confirmatory model indicated a good fit for this factor structure, with a ratio of chi square to degree of freedom of 2.26, goodness-of-fit index of .82, and root-mean-square error of approximation of .06. Given agreement in both samples on the underlying factor structure of family burden, the samples were combined in subsequent analyses.
Analytic strategy
First, patients with and without caregivers were compared on sociodemographic characteristics and the main study measures; chi square or t tests were used. Effect sizes (Cohen's d) also were calculated. Next, preliminary bivariate-level analyses were conducted to identify patient and caregiver variables for inclusion in the multivariate analyses. Measures that had significant bivariate associations at the .05 level or better with at least one of the four burden factors were included in the multivariate models. The neurocognitive and side effects measures did not meet this criterion and were thus excluded. Because data were collected at 53 sites, we tested for site effects but found none; this variable was therefore excluded from further analysis.
Eligible measures were entered into four hierarchical regression analyses to identify correlates of each burden factor. Variables were grouped conceptually, and each group was entered into the regression in a single step. Thus we could evaluate the total impact (change in R 2 ) of that domain as a whole in addition to the significance of individual measures. Patient demographic variables were entered in step 1, and caregiver demographic variables were entered in step 2. Symptom measures were entered in step 3, and quality-of-life and service use measures were entered in steps 4 and 5, respectively.
Results
Comparison of patients by family interview
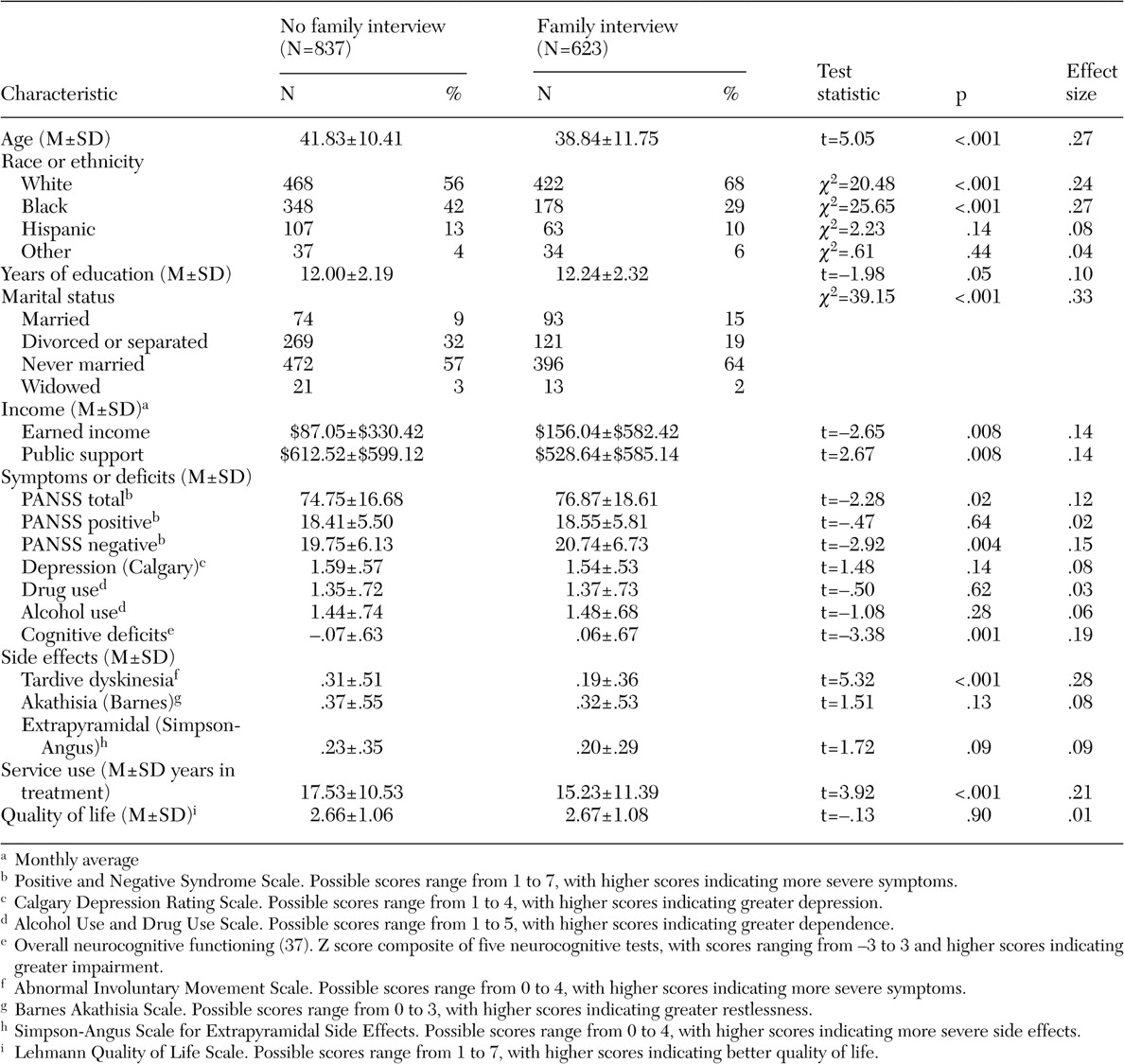
Table 2 shows that patients with families who were interviewed were younger, were more often Caucasian, were less often black, were more likely to be married, had fewer years in treatment, had more years of education, and received less public assistance and more earned income than patients whose families were not interviewed. Patients whose families were interviewed also had higher total and negative symptom scores on the PANSS and less tardive dyskinesia as rated on the AIMS. Their overall neurocognitive functioning was higher. The effect sizes associated with these comparisons were relatively small (.01 to .33) (
Table 2 ).
A logistic regression analysis demonstrated that patients with family interviews differed from others on only six variables (youth, other than black race, higher education level, negative symptom scores, AIMS scores, and total income—earned income plus public assistance).
Multivariate models of burden correlates
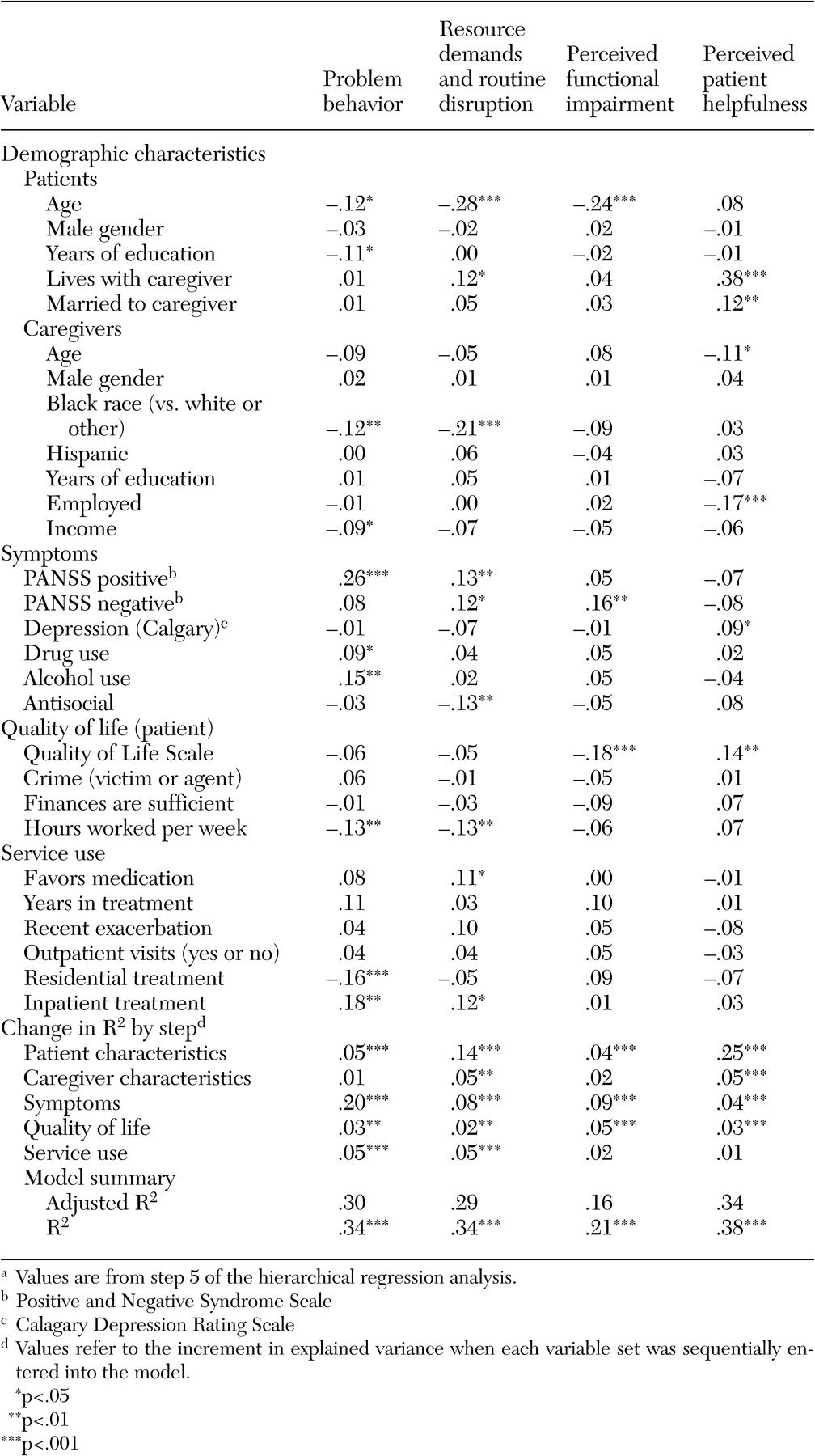
Table 3 presents the results of the multiple regression analyses for the four burden factors. For ease of explication, only the fifth step, which controlled for the effects of all other variables, is presented. The model for each of the four burden factors was highly significant, with R
2 values ranging from .38 for perceived patient helpfulness to .21 for impairment in activities of daily living. We compare below the results of the multivariate models for each class of predictor variables to highlight their differential relationships with the four burden factors.
Patient and caregiver demographic characteristics
Notably, patient and caregiver demographic characteristics, especially age, explained a relatively large amount of variance for the two burden factors related to family and patient interaction—perceived patient helpfulness (30 percent) and resource demands and routine disruption (19 percent)—but contributed relatively little explanatory power to the models for the two factors related solely to patient characteristics—problem behavior and impairment in activities of daily living (6 percent each). Living with and being married to the caregiver were both associated with greater perceived helpfulness, whereas caregiver age and employment status were both inversely related to perceived helpfulness; older, employed caregivers viewed the patient as less helpful. Living with the caregiver also was associated with greater resource (financial) demands or disruption of routine, as was younger patient age. Identifying oneself as black (compared with white or other) was associated with lower levels of resource demands and routine disruption.
Patient symptoms
Patient symptomatology explained modest additional variance in patient helpfulness and resource demands (4 percent and 8 percent, respectively) but explained 20 percent additional variance in problem behavior burden and 9 percent more variance for impairment in activities of daily living. After demographic characteristics, symptoms were the strongest predictor of burden across all four measures. Because the five items with the strongest loading on the problem behaviors factor involved some form of violence (destroying property, for example) (
Table 1 ), the strong association of patient symptoms with this factor is not surprising.
The four burden factors were differentially related to the PANSS negative and positive symptoms dimensions. The problem behavior factor was significantly and positively associated with positive, but not negative, symptoms, whereas the reverse was true for impairment in activities of daily living, which was positively associated with negative but not positive symptoms.
The factor for resource demands and disruption of routine was positively and significantly related to both positive and negative symptoms. The perceptions of patient helpfulness factor, however, was not significantly related to either symptom scale.
Quality of life
Caregivers of patients with higher quality of life reported greater patient helpfulness and lower impairment in activities of daily living. The problem behavior and resource demands factors were not significantly associated with overall quality of life but were strongly associated with the number of hours worked each week—caregivers of patients who worked more hours reported less burden. All together, quality of life contributed modestly (2-5 percent additional variance) to the models for the four burden factors after the analysis controlled for patient symptoms and patient and caregiver demographic variables.
Service use
Treatment in residential settings was negatively correlated with reports of problem behavior burden. By contrast, inpatient treatment was positively associated with this burden factor and with resource demands even after we controlled for patient symptom level, which most likely reflects the short duration of inpatient episodes relative to residential treatment. Somewhat counterintuitively, caregivers of patients who favored medication reported a greater burden from resource demand and routine disruption, even after the analysis controlled for measures of symptom severity and years in treatment. Service use measures explained only an additional 5 percent of variance for problem behavior and resource demands but did not contribute significantly to the other models.
Discussion
This study showed that most family members of a large, heterogeneous sample of patients with schizophrenia reported appreciable and varied strains associated with supporting their relative. Through use of factor analysis and multivariate modeling, we were able to demonstrate that different aspects of family members' experiences supporting the recovery of a relative with schizophrenia are associated with different dimensions of the patient's clinical presentation, treatment, and life functioning. Each of the four burden factors was associated with a different pattern of relationships to the domains examined.
Perhaps the most striking example was seen in the patient symptom domain, where the four burden factors appeared to reflect different aspects of the patient's symptom presentation. Although relatives' experiences of problem behaviors were associated with positive symptoms, their experience of functional impairment was associated with negative symptoms, and their experience of resource demands and routine disruption was associated with both symptom types. The quality of the burdens experienced by family members in relation to negative and positive symptoms thus differed in important respects. The association of negative and positive symptoms with burden is not clear in the literature, as described in the introduction. Discrepancies in the research may be explained in two ways. First, these symptom types may lack sensitivity or specificity to dimensions of burden that differ from those we measured. Second, the discrepancies may be caused by variations in the degree and relative proportion of positive and negative symptoms represented in different clinical samples.
Different burden factors were similarly related to different dimensions of quality of life. The significant associations of family members' perceptions of patient helpfulness and impairment in activities of daily living with overall quality of life suggest that the patient's overall participation in and enjoyment of his or her life may be related to specific facets of caregiving strain. Caregivers may be able to tolerate a greater degree of strain or inconvenience if they perceive that the patient is pulling his or her share of the load. Our results suggest that even when the more florid symptoms of illness have been controlled, caregivers continue to be concerned about the patient's ability to achieve the normal gratifications of social life, work life, and leisure activities.
Although our findings indicate that the patient's use of and attitude toward mental health services contributed to the caregiver's perceived burden, the specific, somewhat counterintuitive pattern of results—that inpatient service use was associated with increased burden after the analysis controlled for patient symptom level on two factors—suggests that additional information is needed to understand these relationships. For example, information regarding the family's needs and expectations from mental health services and service providers for their relatives might help to clarify the ways in which patient service use might best relieve family burden and under what circumstances it might exacerbate perceived burden.
The hypothesis that more intact cognitive functioning would be associated with lower levels of burden was not borne out. Because in this initial study we investigated only cross-sectional associations of neurocognition with burden, our findings do not preclude the possibility that neurocognition may affect caregiver burden over a longer observation period. Cognitive deficits have been associated with higher levels of caregiver burden in studies of dementia (
41,
42,
43,
44 ).
Both patient and caregiver demographic variables were related to burden factors. The finding that younger patients imposed greater burden on families in three of four factors is in line with studies suggesting that patients with schizophrenia have a higher rate of recovery as they grow older (
45 ) and are less dependent on their families. Consistent with the positive association observed between coresidence and resource demands and routine disruption, other studies have found that caregivers rate patients who live with them as more burdensome than do caregivers who reside separately (
8,
46 ).
However, we found that the greater demands on resources and disruption of routines experienced by caregivers of coresiding patients have to be balanced against their greater perceptions of patient helpfulness. Although the financial demands of caregiving are among the most onerous, contributions from the patient may help to offset the emotional as well as practical repercussions of this aspect of caregiving. Patients who live with the caregiver also provide emotional support and companionship. The identification of a perceived patient helpfulness factor in this study is in line with recent research findings underscoring the importance of the full relationship between patient and caregiver, not just the emotional and practical stress experienced by the caregiver (
47,
48,
49,
50 ).
A strength of this study is the relatively large samples of black and Hispanic caregivers, which provided sufficient power to examine racial and ethnic differences. The finding that black caregivers reported lower levels of both problem behavior and resource demand burden is consistent with prior studies of caregiving in dementia and severe mental illness, which found that black caregivers reported lower levels of caregiving strain and depression and higher levels of self-efficacy and mastery compared with white caregivers (
51,
52,
53,
54 ).
Because studies of burden have traditionally focused on the impact of patient symptoms, this study aimed to explore the contribution of other important patient attributes and behavior on their relatives' experience of caregiving strain. In particular, given the recent attention to the role of neurocognition in schizophrenia, including the argument that schizophrenia is a "disorder that manifests itself primarily in cognition" (
55 ), we sought to examine the influence of neurocognition on burden. It is therefore striking that patient symptoms emerged as the most important contributor to family burden, after we controlled for patient and caregiver sociodemographic variables. Although quality of life and service use each contributed to the family's experience of burden, these contributions were relatively modest compared with the contribution of patient symptoms.
Although this study may represent the largest cohort of family caregivers of patients with schizophrenia to be studied, our findings must be interpreted with some degree of caution. First, because only about half of the patients' family members participated, we cannot generalize to the larger sample of family caregivers of people with schizophrenia. Because the patients whose caregivers participated were more symptomatic than patients whose relatives did not, the degree of burden reported may over- or underrepresent the degree of burden experienced. Second, a clinical trial sample itself may not be representative of the total population of people in treatment or the total population of people with schizophrenia. Third, these data constitute the baseline information from a longitudinal study, and the associations reported are thus cross-sectional and cannot be used to infer causality. Future reports will focus on the relationship of caregiver burden to patient symptoms, quality of life, and service use over time, as well as on the consequences of burden as they relate to other caregiver and patient outcomes.
Clinical interventions for families are most often delivered when the patient is acutely ill, often as part of an inpatient stay. Our results underscore the need for continued intervention with family members even after the patient's symptoms have stabilized. To address this need, programs such as multiple-family group therapy (
56 ) and the Family-to-Family program developed by the National Alliance on Mental Illness (
57 ), which offer longer-term support for caregivers in a relatively cost-effective (group) setting, might be offered as part of a menu of family services in psychiatric outpatient or rehabilitation settings. Involvement of families in their relatives' ambulatory care has been associated with improvement in patient outcomes as well, including higher employment rates and fewer negative symptoms (
58 ). Improved outcomes for patients associated with family involvement in their treatment may be related to reductions in burden, given that lower levels of burden have been shown to predict better patient outcome (
59 ).
Conclusions
Interventions for family members have frequently focused on modifying the family's affective response and behavior toward the patient. Such interventions are based on findings that expressed emotion, an index of the family's emotional response to the patient, predicts relapse (
60 ). Our findings on patient helpfulness underscore the importance of educating consumers, as well as caregivers, on the importance of their contributions to the household in reducing the burdens their illness imposes on the caregiver and maintaining a more positive affective climate.
Our findings are thus consistent with the recovery philosophy of expanding treatment options beyond symptom reduction (
61,
62 ). They suggest that interventions to support and develop the consumer's ability to contribute to the household and management of his or her own care, such as skills training (
63 ) or wellness recovery (
64 ), are most likely to meet the needs and enhance the quality of life of people with mental illness and their families.
Acknowledgments
This article is based on results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project, supported by grant NO1-MH-90001 from the National Institute of Mental Health (NIMH). The project was carried out by principal investigators from the University of North Carolina, Duke University, the University of Southern California, the University of Rochester, and Yale University in association with Quintiles, Inc.; the program staff of the Division of Interventions and Services Research of NIMH; and investigators from 56 sites in the United States (CATIE Study Investigators Group). AstraZeneca Pharmaceuticals L.P., Bristol-Myers Squibb Company, Forest Pharmaceuticals, Inc., Janssen Pharmaceutica Products, L.P., Eli Lilly and Company, Otsuka Pharmaceutical Co., Ltd., Pfizer, Inc., and Zenith Goldline Pharmaceuticals, Inc., provided medications for the studies. The Foundation of Hope of Raleigh, North Carolina, also supported this work. Details about the CATIE Study Investigators Group are available at www.catie.unc.edu/schizophrenia/locations.html#clinicalsitelocation list.