The etiopathogenesis of schizophrenia has remained elusive. Several findings have, however, suggested that organic factors contribute to the pathogenesis of the disease. Among these, hereditary factors
(1), neurodevelopmental disturbances
(2,
3), and immunological aberrations
(4–
7) have been proposed to be of significance. The attractiveness of the dopamine theory has been refreshed by reports on abnormal expression of the D
4 and D
5 receptors
(8,
9). Abnormalities in the sites of serotonin (5-HT) uptake
(10) and 5-HT receptors
(11) have also been implicated. The action of atypical antipsychotic drugs such as clozapine and risperidone have been supposed to be related to their 5-HT
2 antagonism
(12). Modern neuroimaging techniques have disclosed low volume
(13–
15) and gray matter deficits
(16,
17), especially concerning limbic structures in the medial temporal lobe surrounding the temporal horn
(18,
19), in the brains of schizophrenic patients. Skewings of circulating and CSF cytokine levels and production have been detected; the affected cytokines include interleukin 2 (IL-2)
(20,
21), IL-6
(22), and tumor necrosis factor alpha
(23).
These findings have raised the question of whether the abnormalities originate from developmental, degenerative, inflammatory, or immunoactive processes in the central nervous system (CNS).
Experimental models of nerve injury have revealed the critical role of macrophages in both degenerative and regenerative actions in the nervous system
(24,
25). In certain neurological diseases, where patients suffer from neuronophagia, the activity of resident macrophages (from microglial or perivascular origin) seems to contribute to the disappearance of neurons
(26).
RESULTS
Cytological examination of the CSF from the 35 psychotic patients revealed that they had a significantly higher proportion of cells morphologically classified as mononuclear phagocytes/macrophages than was present in the CSF of the comparison subjects (F=47.34, df=1, 79, p<0.0001). The total CSF cell count was not, however, significantly higher in the patient group.
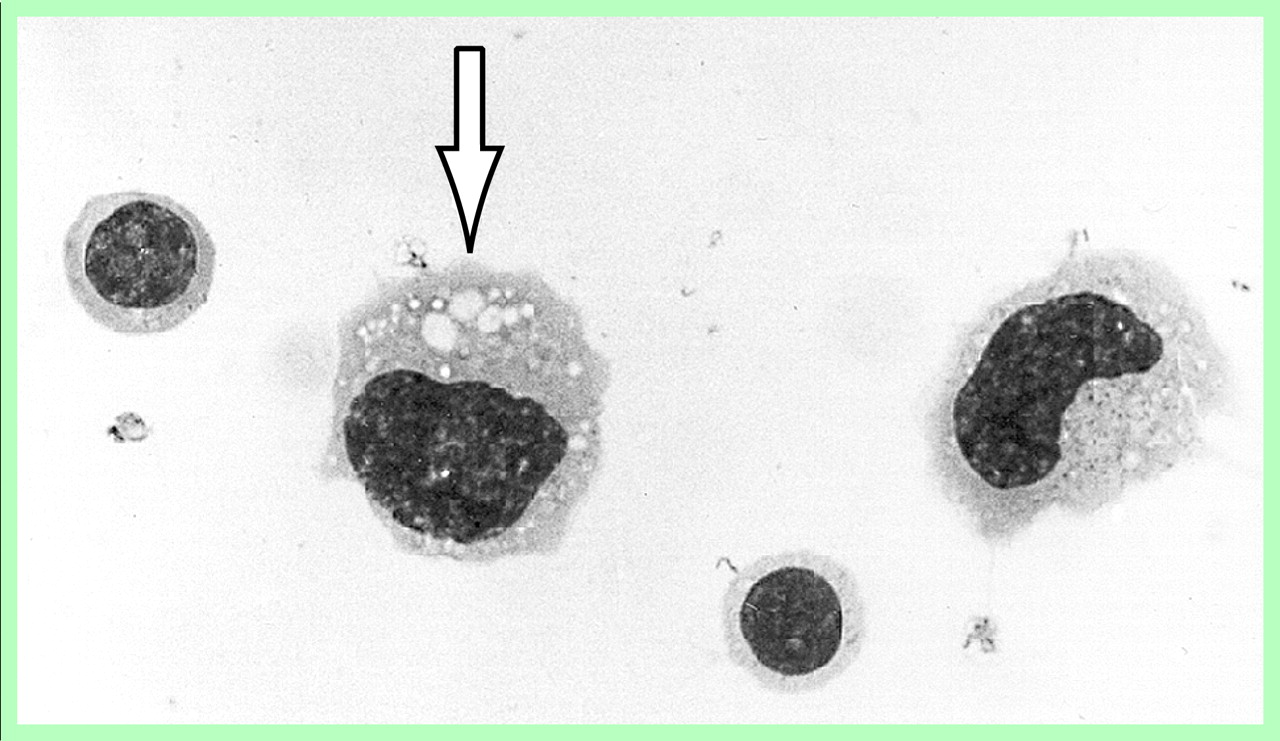
The cytological details of the phagocytes frequently included a kidney-shaped or lobulated nucleus and a voluminous cytoplasm with numerous small vacuoles. Overt lipophages with larger cytoplasmic vacuoles, typically seen in the CSF after acute brain injuries, for instance, were rarely seen (
Figure 1).
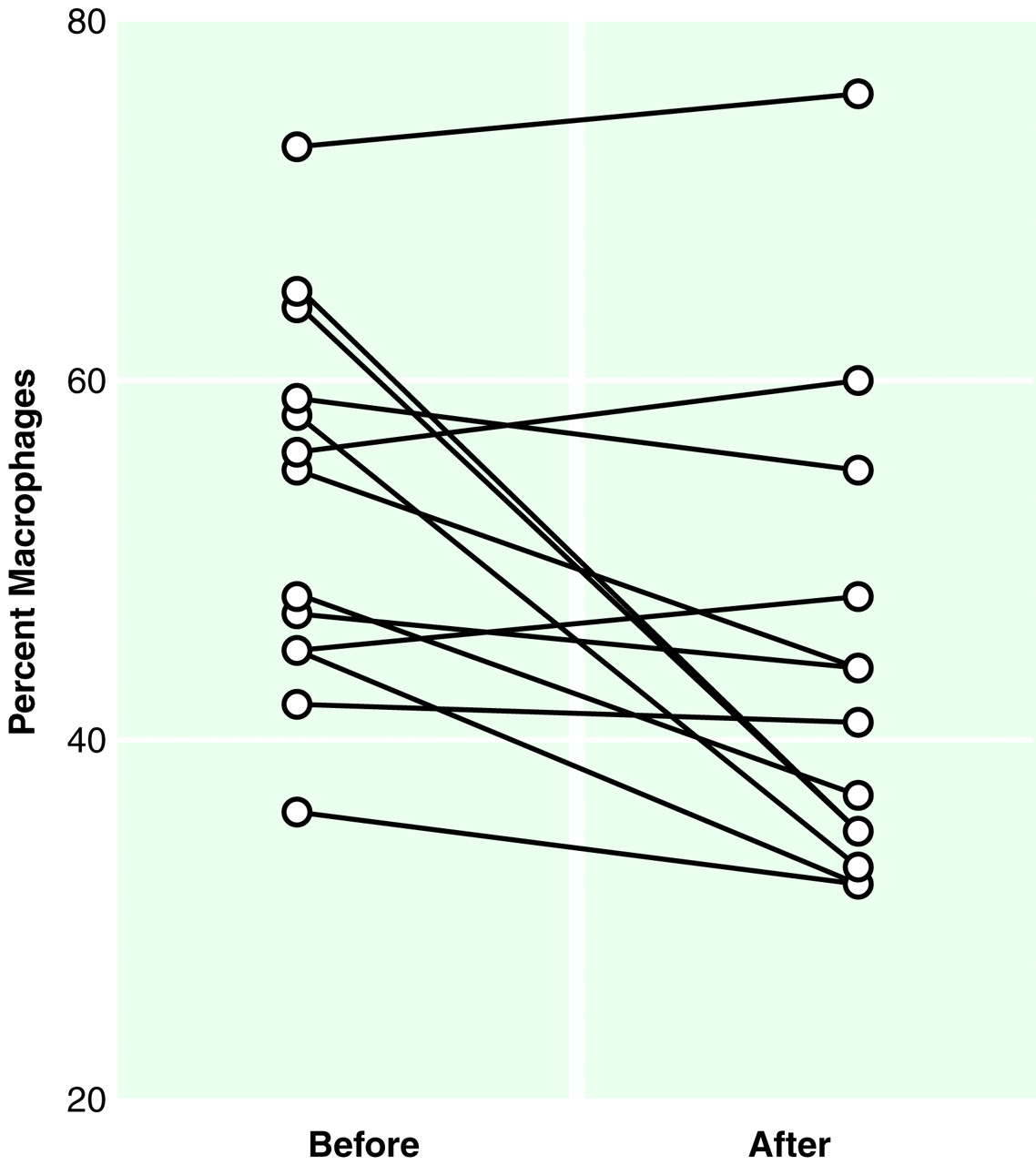
A tendency toward normalization of the cytological picture (i.e., decrease in the proportion of macrophages) was observed in the CSF of the 13 psychotic patients who took part in the follow-up section of the study (Wilcoxon matched pairs test, rank transforms, and repeated measures ANOVA: F=7.83, df=1, 12, p<0.05) after a few weeks of treatment with typical neuroleptics (
Figure 2).
DISCUSSION
There is a growing body of evidence that genetic susceptibility significantly contributes to the etiology of schizophrenia
(33). Although the schizophrenia susceptibility genes are still to be identified, several reports have indicated the location of such gene(s) on chromosome 8
(34) or on the short arm of chromosome 6
(35). Incomplete penetrance and environmental forms of phenocopies still leave room also for gene-environment interactions
(36,
37), and some associations of human lymphocyte antigens (HLAs) in schizophrenia have been detected
(38–
40), suggesting that immune mechanisms may contribute to the etiology of the disease. Viewed from the angle of these processes, the accumulation of macrophages in the CSF during acute schizophrenia reported here may reflect a genetically transformed immune response to (or may be caused by) environmental factors.
Abnormalities of the gross anatomy of the brain are frequently found in schizophrenia. Both autopsy studies and modern brain imaging have revealed a loss of brain substance, in particular from the fronto-occipital areas, and enlargement of the CSF space
(13–
19). These abnormalities may signify dysregulated brain development in schizophrenia. In addition, there are reports of a correlation between the extent of macroscopic brain abnormalities and the duration of the psychotic disease, which implicates a contribution by some chronic degenerative process in the CNS
(41). Macrophage dominance is a frequent finding in CNS injuries
(42). Consequently, our detection of an excess of vacuolated macrophages in the CSF of patients with schizophrenia may reflect some organic brain destruction of subchronic or chronic type and relate to the neuroradiologically demonstrated low brain volume in schizophrenia.
Most of the macrophages found in the CSF are considered to be of microglial derivation
(43,
44). Microglia, which originate from hematopoietic stem cells
(45,
46), constitute the main source for the macrophages accumulating in lesions of the CNS. In addition to their phagocytic capacity, microglial cells contribute to the immune network in the CNS by expressing HLA-DR molecules
(47) and acting as antigen-presenting cells
(48). The absence of neutrophil recruitment and the delay in the increase in macrophage or microglial cells show that the CNS differs from other sites in the body with regard to the kinetics and nature of the myelomonocytic cell responses
(49). Coculturing of microglia with T lymphocytes results in clustering of T cells around the microglia and initiation of mixed lymphocyte reaction
(50), and activated T lymphocytes induce the cells of the macrophage lineage to produce pro-inflammatory cytokines
(51). Moreover, the CNS mononuclear cells express receptors for neurotransmitters and may therefore functionally bridge the CNS and the immune system
(52,
53). The macrophage dominance in the CSF of psychotic patients detected in this study may reflect activation or mobilization of the microglial cells, or both, and may also link the previous reports on T lymphocyte deviations and cytokine aberrations in schizophrenia.
Given that 5-HT has been found to act as a modifier of dopamine responses
(54) and as an activator of macrophages
(55), it is tempting to speculate that abnormal monoamine metabolism in acute schizophrenia may contribute to the mobilization or activation of microglia leading to the relative macrophage dominance in the CSF. Our finding of neuroleptic-induced normalization of the CSF cytology in many patients lends support to this hypothesis.
A macrophage dominance over lymphocytes, as in the CSF of the adult schizophrenic patients in this study, is also typical in the CSF of newborn infants, and normally the cell profile gradually transforms with age into that of normal adults (i.e., lymphocyte dominance of 60%–80%)
(27). This phenomenon may have bearing on the neurodevelopmental theories of schizophrenia
(2,
3), especially because programmed cell death (apoptosis) and axonal pruning are thought to be essential actions in neurodevelopmental sequences, are substantially affected by macrophages and microglia, and are influenced by both genetic and environmental factors
(56).
Macrophages have numerous effects on the CNS mediated by cytokines and neurotoxins
(56), and further CSF analysis of, for example, pro- and anti-inflammatory cytokines and excitatory amino acids will be needed to further clarify the more detailed role of macrophages and microglia in the pathogenesis of schizophrenia.