Schizophrenia is a chronic, debilitating mental illness that affects approximately 1% of the population and is typically characterized by positive symptoms such as auditory hallucinations and thought disorder. During the past decade, advances in neuroimaging methods to study the brain have resulted in a better understanding of the neuropathological basis of schizophrenia. Yet the relationship between hand dominance and asymmetry of brain pathology in this disorder has attracted little attention. There is, however, a large literature pointing to abnormalities in the temporal lobe, especially left-sided pathology
(1–
3). Anomalies in the temporal lobe and the superior temporal gyrus have been identified in right-handed schizophrenic men on the basis of evidence from functional
(4,
5), structural
(6), and histological
(7,
8) studies. Moreover, positive symptoms in right-handed schizophrenic men have been linked to magnetic resonance imaging (MRI) studies showing abnormalities in the left anterior
(9) and left posterior
(10,
11) superior temporal gyrus. With respect to handedness, several investigations have shown that left-handed and right-handed male schizophrenic patients can be dissociated on the basis of anatomical, cognitive, and functional differences. For example, left-handed schizophrenic men have larger lateral ventricles and more cognitive deficits than do right-handed schizophrenic men
(12). Electrophysiologically, topographic asymmetries of the P300 peak are right-lateralized in the temporal region in left-handed schizophrenic men, whereas there are left-lateralized P300 asymmetries in right-handed schizophrenic men
(5,
13). As a whole, evidence supports left hemisphere pathology in right-handed schizophrenic men. Evidence is emerging, however, that both hemispheres or the right hemisphere are affected in left-handed schizophrenic men.
Given that handedness is related to cerebral organization
(14) and that left hemisphere abnormalities have been linked to positive symptoms in right-handed schizophrenic men, findings of altered asymmetry in left-handed schizophrenic men would contribute additional evidence that underlying neuropathology may be linked not only to mechanisms determining cerebral dominance, but also to handedness in schizophrenic men. Because of the limited number of studies in this area and the reported left-sided abnormalities in right-handed schizophrenic men, we were interested in whether left-handed schizophrenic men would show reversed or more symmetric low volumes in the superior temporal gyrus than are present in right-handed schizophrenic men and also whether the anatomical abnormalities would correlate with thought disorder. We hypothesized that left-handed schizophrenic men would have significantly lower volumes in the right and left superior temporal gyrus or in the right superior temporal gyrus and that these volume differences would be correlated with formal thought disorder.
RESULTS
The volumetric analyses were corrected for intracranial volume to normalize for differences in head size. Accordingly, relative volumes were obtained by dividing the volume of the intracranial contents and multiplying by 100. All statistical analyses were based on relative volumes. The effects of region and laterality on the volumes of superior temporal gyrus structures were analyzed by using repeated measures analysis of variance (ANOVA), which was followed by Scheffe planned comparisons in which the p value was set at ≤0.02. Spearman’s rho (r
s) was used to correlate thought disorder scores with left and right anterior, posterior, and total superior temporal gyrus regions. Asymmetry coefficients were computed for each superior temporal gyrus region (right – left) / 0.5 × (right + left)
(23). Kendall’s tau-b was used to correlate asymmetry measures with superior temporal gyrus regions because of tie values. All p values are based on two-tailed tests.
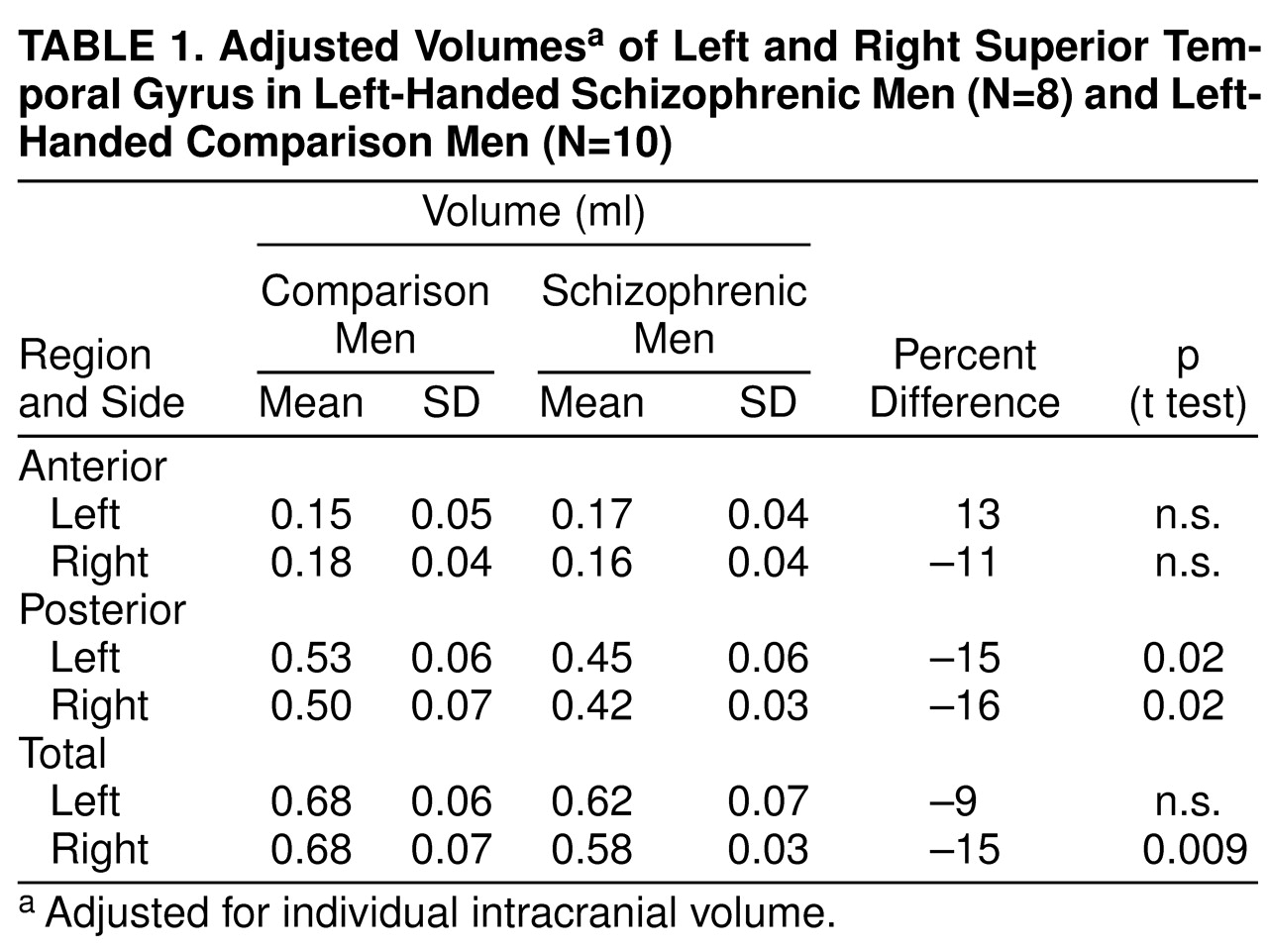
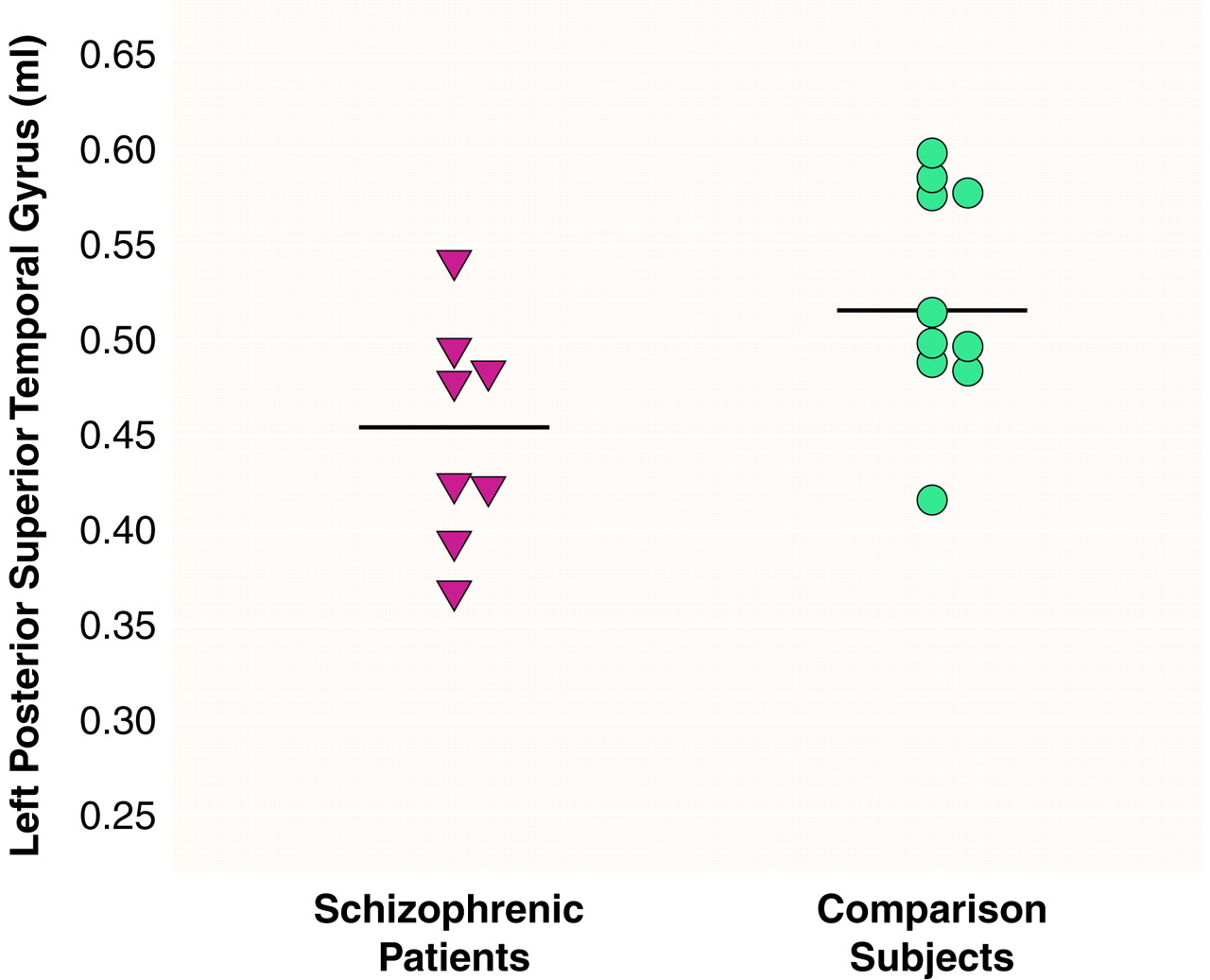
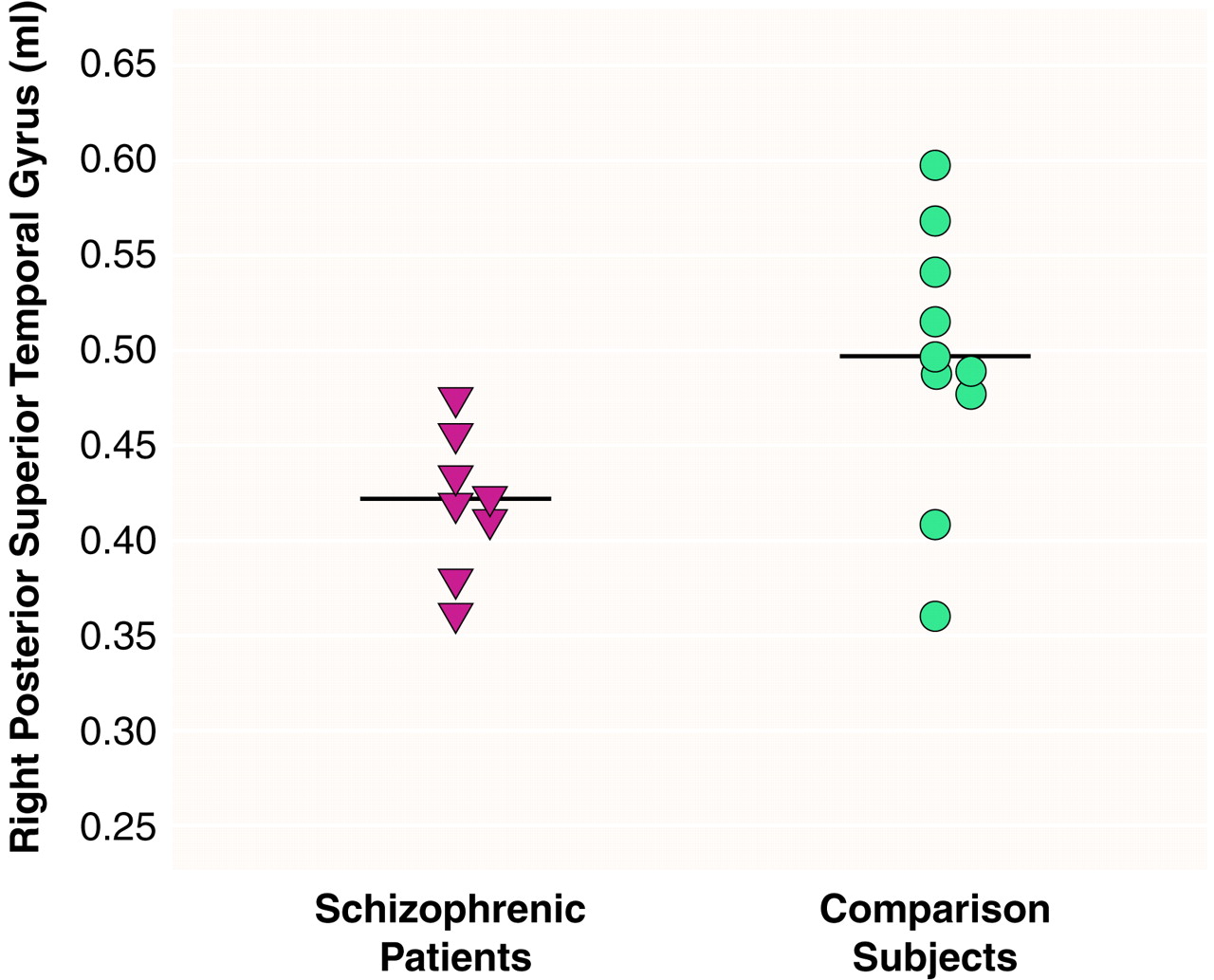
There were significant effects of diagnosis (F=6.83, df=1, 16, p<0.01) and region (F=290.65, df=2, 15, p<0.001) and a significant diagnosis-by-region interaction (F=4.43, df=2, 15, p<0.05), indicating different volume patterns in the patients and comparison subjects. The patients showed significantly smaller volumes bilaterally in the posterior superior temporal gyrus: 15% less volume in the left posterior region (t=2.68, df=16, p<0.02) (
table 1 and
Figure 2) and 16% less volume in the right posterior region (t=2.69, df=16, p<0.02) (
table 1 and
Figure 3). The patients also showed significantly lower volume in the right total superior temporal gyrus (t=2.95, df=16, p<0.01) but not in the left total superior temporal gyrus (
table 1). Contrary to our expectations, the left-handed patients showed a positive correlation between severity of thought disorder and right anterior superior temporal gyrus volume (r
s=0.87, N=7, p=0.005) (
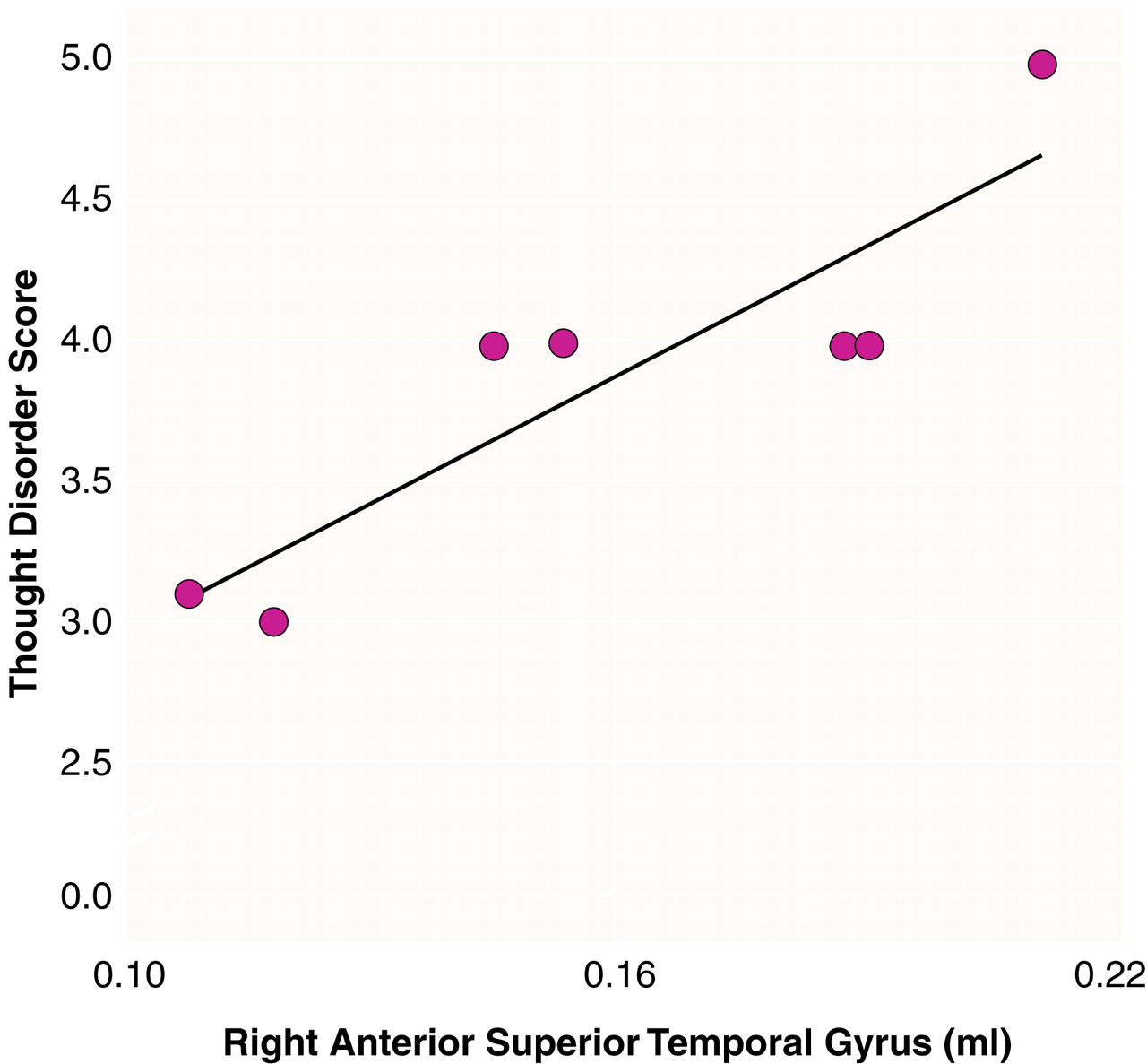
Figure 4).
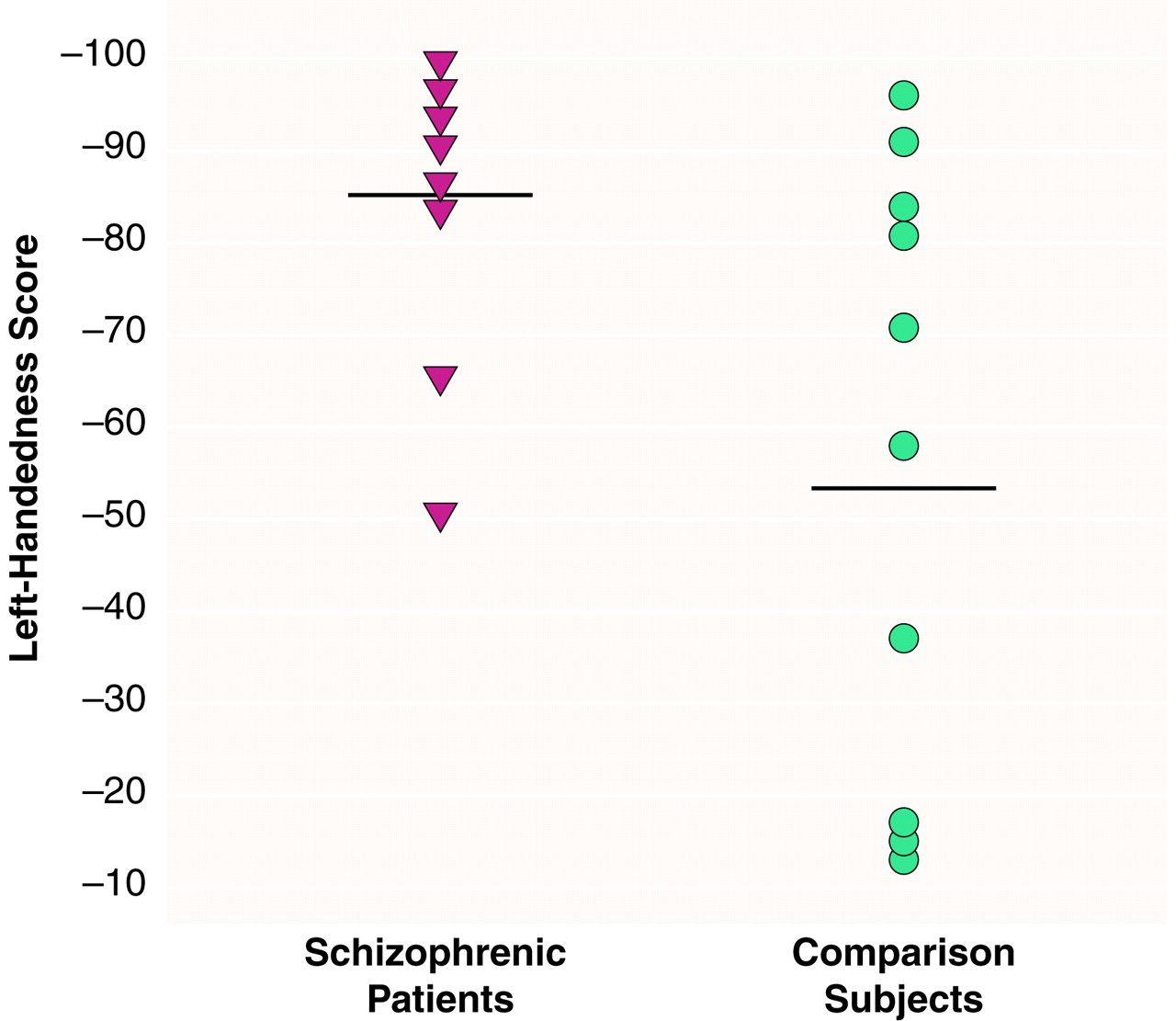
The patients were significantly more left-handed than the comparison men (t=2.17, df=16, p<0.05) (
Figure 1). Removing three comparison subjects whose handedness quotients were below –20 resulted in the same general direction of differences, e.g., a significant diagnosis-by-region interaction (F=4.69, df=2, 12, p<0.05), indicating that the handedness difference did not affect the results. The patients also had significantly less intracranial content than the comparison men (t=2.24, df=16, p<0.05). Two of the three comparison subjects who scored less than –20 on the handedness scale also had the highest values for intracranial content. Without their data, there was no significant difference in intracranial content between the groups.
Analyses of asymmetry coefficients (absolute values) yielded three significant correlations. First, the patients showed a significant negative correlation between asymmetry in the anterior superior temporal gyrus and volume of the right anterior region (tau-b=–0.57, N=8, p<0.05), indicating that as asymmetry decreases the right anterior superior temporal gyrus gets larger. Second, the comparison men showed a significant negative correlation between asymmetry in the anterior superior temporal gyrus and volume of the left anterior region (tau-b=–0.50, N=10, p<0.05), demonstrating that the left anterior superior temporal gyrus gets bigger as asymmetry decreases. Third, there was a significant positive correlation for the comparison men between asymmetry in the posterior superior temporal gyrus and the volume of the left posterior region (tau-b=0.51, N=10, p<0.05), showing that the left posterior superior temporal gyrus gets larger as asymmetry increases. There were no other significant correlations for either group.
DISCUSSION
To our knowledge, this is the first MRI study to investigate superior temporal gyrus abnormalities in left-handed schizophrenic men. Our results, which showed bilaterally low volume in the posterior superior temporal gyrus and low right-side volume in the total superior temporal gyrus, differ from the numerous findings of unilaterally low volume in the left superior temporal gyrus of many right-handed schizophrenic men
(6,
9–
11). Similarly, our finding of a positive correlation between thought disorder and greater volume of the right anterior superior temporal gyrus is opposite to previously reported negative correlations between positive symptoms and low left superior temporal gyrus volume in right-handed schizophrenic men
(9–
11). These differences may reflect abnormalities in neuropathology that are part of the schizophrenic disorder in men who are left-handed, a relationship that has been hypothesized
(24) but heretofore lacking in empirical data.
In normal subjects, 25%–85% of left-handers show nonstandard asymmetry of the superior temporal gyrus, i.e., bilateral symmetry (left same as right) or reversed asymmetry (left less than right) compared to the standard asymmetry (left greater than right) found in many right-handers
(25–
27). Our left-handed comparison subjects showed nonstandard asymmetry in the total (left same as right) and anterior (right greater than left) superior temporal gyrus. Our findings are consistent with those from other studies of left-handed men
(25–
27).
MRI studies of the superior temporal gyrus in right-handed schizophrenic patients have shown low volume
(9–
11,
28–
30) and no differences
(31,
32). The left-lateralized low volumes in right-handed schizophrenic patients (9–11) may be a neuropathological expression of the standard asymmetry (left greater than right) shown for right-handed normal men in MRI studies
(25–
27) and postmortem studies
(23). Similarly, the bilateral and right-lateralized low volumes in our left-handed schizophrenic patients may be a neuropathological expression of nonstandard superior temporal gyrus asymmetry (bilateral or reversed), consistent with our left-handed comparison subjects and subjects in other studies
(25–
27). Thus, it may not be that schizophrenia is lateralized left or right but, rather, that its pathology is related to handedness
(33), affects cortical volume, and alters degree and direction of asymmetry
(34).
The correlation between greater thought disorder and greater volume in the right anterior superior temporal gyrus in the left-handed patients is contrary to our prediction that thought disorder would be associated with lower superior temporal gyrus volume (left or right). A similar finding was reported for right-handed schizophrenic patients but without measures of handedness
(32), a factor that may have influenced those results. In our left-handed patients, the positive correlation may be explained by the abnormal asymmetry in the anterior superior temporal gyrus. We suggest that the abnormal asymmetry, driven by the relatively lower volume of the right anterior region and preservation or even greater volume of the left anterior region, may reflect the disturbance of the normal anatomical relationships and consequently may be associated with greater thought disorder. The anterior and posterior portions of the superior temporal gyrus are linked functionally and by anatomical connections. The positive correlation between thought disorder and volume of the right anterior region may, therefore, reflect a flawed reorganization of the right superior temporal gyrus functionality and its anatomical substrate occurring as a result of abnormalities throughout the posterior portion of the superior temporal gyrus, both left and right.
Our findings provide a broader context for the research into left-lateralized superior temporal gyrus abnormalities in right-handed schizophrenic men by demonstrating bilateral and right-lateralized abnormalities in left-handed schizophrenic men. With the caveat that our findings are based on a small number of subjects, these data and those from previous studies
(5,
13) support the view that handedness is a strongly differentiating variable in studies of schizophrenia. However, other variables, such as gender, also need to be studied, particularly since left-handedness appears more in males than in females
(35) and gender differences have been reported in MRI studies of schizophrenia
(36,
37).
In conclusion, our findings show that handedness is a major contributor to differences in the brain pathology of schizophrenia. Taken together with language-related abnormalities in the superior temporal gyrus of right-handed schizophrenic men, our data are consistent with the proposal that the pathology of brain asymmetry is related to cognitive dysfunction and handedness in schizophrenic men. These findings are important because they afford a possible clue to underlying mechanisms of schizophrenia, namely that this disorder may be linked to neurodevelopmental factors influencing language, cerebral asymmetry, and handedness.