Schizophrenia is associated with multiple indicators of disease liability, including functional and structural brain abnormalities, sensory processing deficits, and neuromotor abnormalities. In addition, particularly pronounced impairments in cognition have been well documented and are thought to be a core feature of the illness (
1–
5). Assessed by various neuropsychological tests, cognitive impairments in schizophrenia are a large and pervasive facet of the illness (
6–
8), enduring through the lifespan (
9). Not only do these impairments reduce a patient's ability to reason, plan, retain information, and problem-solve, but they also have been shown to impair quality of life and to compromise everyday functioning (
10,
11) and are considered to be directly associated with the long-term disability that represents one of the major public health costs associated with schizophrenia. A primary question that remains to be answered, however, is: At what point in the illness do cognitive impairments begin to influence ability to function? While evidence clearly indicates that cognitive deficits in patients with chronic illness are directly related to functional outcome, independently of positive symptoms (
10,
11), the interaction between these two domains before the onset of illness is unknown. The issue is whether cognition affects functioning before the onset of psychosis or whether this association is an outcome of illness. Such developmental information is becoming increasingly important for designing effective early treatments to improve functional outcome.
Studies of adolescents and young adults at high risk for developing psychosis have demonstrated that neurocognitive impairments are detectable before the onset of psychotic symptoms. For example, in a preliminary Recognition and Prevention Program study, Lencz et al. (
12) reported that individuals at clinical high risk for developing psychosis performed one standard deviation below the healthy comparison group mean on tests measuring verbal memory, executive function, language, and processing speed. Additionally, several recent studies investigating neurocognitive function in patients at high risk for psychosis reported impairments in verbal memory/learning, executive function, and processing speed (
13–
15). While these studies involved a wide range of assessment methods, and although reports of specific neurocognitive impairments have not been wholly consistent, collectively the findings support the idea that cognitive abnormalities are detectable well before the onset of psychosis.
Preliminary evidence also supports the view that in addition to cognitive impairments, functional impairments are also rooted early in development. Pre-illness social problems and school difficulties have been consistently reported in studies of those at genetic high risk and in cohort studies. In addition, two prospective studies of patients at clinical high risk for psychosis (
16,
17) indicated that, relative to healthy comparison subjects, patients at high risk have significant impairments in maintaining social/interpersonal relationships and managing academic and work tasks, comparable to individuals with first-episode and multi-episode schizophrenia (
16). These findings are consistent with the increasing emphasis on functional decline as a critically important outcome that parallels conversion to psychosis and with the growing notion that psychosis and long-term functional disability are equally important targets for prevention.
As noted, the relationship between neurocognitive and functional impairments before the onset of psychosis is unknown. To date, only one study has examined this relationship in adolescents at high risk for psychosis, and it reported a positive relationship between neurocognition and functional impairments (
18). The findings are difficult to generalize, however, given the small sample size and lack of a healthy comparison group. In this study, we focused especially on the relationship between neurocognition and early functional behavior before the emergence of psychosis. We examined a large sample of treatment-seeking adolescents and young adults at clinical high risk for psychosis. A healthy adolescent group was used as a comparison group to determine the degree to which patients at high risk deviate from normal. A broad neuropsychological battery representing multiple cognitive domains was administered to all participants. In addition, social and role (academic or occupational) functioning was measured with the Global Functioning: Social and Role scales (
17), developed specifically for use with adolescents and young adults at clinical high risk for developing psychosis. We addressed the following questions: What is the extent of cognitive and functional impairment before the onset of psychosis? How does impaired cognition affect and contribute to poor functioning in adolescents at clinical high risk for psychosis? How do positive symptoms combine with neurocognition in explaining functioning?
Method
Study Participants
We enrolled 127 patients who met criteria for “clinical high risk, positive,” derived from the Scale of Prodromal Syndromes (
19,
20). Inclusion criteria were based on the presence of one or more attenuated positive symptoms rated as moderate, moderately severe, or severe (scores of 3, 4, or 5 on a scale of 0–6) on the Scale of Prodromal Syndromes without reaching the level of psychosis. Positive symptoms included unusual thought content, suspiciousness, grandiosity, perceptual abnormalities, and disorganized communication. A score of 6 (severe and psychotic) on any item was an exclusion criterion. Participants in the clinical high-risk group are broadly comparable to those considered to have prodromal symptoms in most other studies. Participants were referred to the Recognition and Prevention Program by affiliated outpatient and inpatient psychiatry departments, local mental health providers, and school psychologists or counselors or were self-referred.
We enrolled 80 healthy volunteers as comparison subjects. Healthy comparison subjects were recruited through announcements in local newspapers and within the medical center during the same period as the patients.
All participants had to be English-speaking and between the ages of 12 and 22 years. Exclusion criteria for all participants included any of the following: a schizophrenia spectrum diagnosis, such as schizophrenia, schizoaffective disorder, schizophreniform disorder, or delusional disorder, as assessed by the semi-structured interview of the Schedule for Affective Disorders and Schizophrenia for School-Age Children, Epidemiologic Version (
21); a medical or neurological disorder that could affect brain functioning; and estimated IQ below 70. Healthy comparison subjects were excluded if they had a first-degree relative with a diagnosed axis I psychotic disorder.
Written informed consent was obtained from patients 18 years of age or older and from parents of patients under 18, with written assent from the patient. The research protocol was approved by the Institutional Review Board at North Shore-Long Island Jewish Health System. The data reported here were collected as part of the larger Recognition and Prevention Program, an ongoing longitudinal investigation initiated in 1998 and funded by the National Institute of Mental Health in 2000. In this article, we report baseline data on all participants who completed the phase 1 neuropsychological test battery (January 1998–February 2008) and were rated on the two global functioning rating scales.
Baseline Neurocognitive Assessment
A comprehensive battery of tests, taking approximately 3.5 hours, was administered to all participants at study entry. Assessors were at the master's level or above and were trained in the administration and scoring of all tests. Estimated full-scale IQ scores were derived from the vocabulary and block design subscales of the WISC-III (
22) for patients under age 16 and from the same subscales of the WAIS-R (
23) for those age 16 and older. All participants received the reading subtest of the Wide-Range Achievement Test 3 (WRAT-3;
24), which would provide an estimate of premorbid intellectual levels in the patient group. In addition to the intelligence scales, the battery included neuropsychological tests that assessed eight cognitive domains: processing speed, verbal memory, executive function, working memory, visuospatial processing, motor speed, sustained attention, and language. Domain construction was based on several factors: rational criteria derived from the literature on clinical neuroscience and neuropsychology; previous work by our group and others that demonstrated the content validity of the domains (
25); and findings of separable factors in the schizophrenia cognitive architecture, including processing speed (
26,
27). As shown in
Table 1, internal reliability (Cronbach's alpha) for these domains was good, thus minimizing the possibility of identifying spurious differences across domains (
28,
29).
Baseline Clinical Assessment
Social and role functioning was assessed using the Global Functioning: Social and Role scales (
17). These scales, which provide clinician-rated single overall scores, are similar in scope and design to the Global Assessment of Functioning Scale and the Social and Occupational Functioning Assessment Scale (
30). However, the new Global Functioning scales differ substantially from both of these in that they represent parallel (one targeting social, the other role) well-anchored scales that take age and phase of illness into account, enabling social and role functioning to be studied as independent domains not confounded by clinical symptoms. The Social scale assesses quantity and quality of peer relationships, level of peer conflict, age-appropriate intimate relationships, and involvement with family members. The Role scale rates level of performance in primary role: school, work, or homemaker. For both scales, scores range from 1 to 10, with 1 indicating extreme dysfunction and 10 indicating superior functioning.
Statistical Analysis
All analyses were conducted using SPSS, version 16.0 (SPSS Inc., Chicago). Before domain scores were computed, raw test scores were transformed into standard z scores using the age-stratified means and standard deviations of the healthy comparison subjects to control for age-related change in cognitive performance. When applicable, tests were reverse-scored, so that lower scores always reflected worse performance. Domain scores were then computed by averaging each participant's z scores on tests assessing the same neurocognitive domain (
Table 1); z scores for each domain were then restandardized using the mean and standard deviation of the domain scores of the healthy comparison group. A composite of global neurocognitive performance was calculated by creating a mean of the eight domain scores. The numbers of patients contributing to the analyses of the various tests varied slightly because of missing data.
To examine whether patients at clinical high risk for developing psychosis have different neurocognitive test profiles as compared to healthy comparison subjects, the eight neurocognitive domain scores were used as dependent variables in a multivariate analysis of covariance (MANCOVA) with group as a between-subject factor and neurocognitive domain scores as within-subject factors. MANCOVA was used to assess the effects of gender and race since the two groups differed on these variables. Deviations from flatness in the clinical high-risk cognitive profile, if suggested by a significant main effect of overall cognitive performance and a significant interaction between cognitive performance and group, were assessed by contrasting the mean for each individual domain with the mean of all other domains, using paired t tests. This procedure allowed us to identify possible specific impairments relative to each of the other domains, rather than a generalized impairment affecting all cognitive domains in the clinical high-risk group, psychometric characteristics of the tests notwithstanding.
Next, we examined neurocognitive performance independent of performance in other cognitive domains by using analysis of covariance (ANCOVA). Individual ANCOVAs were used to examine differences in neurocognitive performance by domain, with group as a between-subject factor, neurocognitive domain scores as dependent variables, and gender and race as covariates. The strength of the variables examined by ANCOVA was evaluated using Cohen's f statistic (
31).
Two sets of multiple linear regressions with forward (stepwise) inclusion were constructed to predict the independent contributions of neurocognitive performance to functional impairment in patients at clinical high risk for psychosis. The first set of regression models examined the association between global neurocognition and functioning, with the global neurocomposite score as an independent variable and the Global Functioning: Social and Role scores as dependent variables. After accounting for global neurocognition, the second set of regression models examined the relationship between domain-specific neurocognitive performance and functional impairment, with neurocognitive domain scores as independent variables and social and role scores as dependent variables, controlling for gender and race. Follow-up stepwise linear regressions were constructed to determine the relative contributions of neurocognitive performance and positive symptom severity in explaining the variance in social and role functioning.
Logistic regression analysis was used to test whether neurocognition could be used to accurately determine functional status at study entry. Social and role scores were transformed into dichotomous variables in which poor functioning was labeled 1 and good functioning was labeled 0 (scores ≤6 were rated as poor functioning and scores >6 as good functioning) (
32). One set of binomial logistic regression models with forward (stepwise, likelihood ratio method) inclusion was performed with the neurocognitive domain scores of both groups as independent variables and baseline functional status (social and role) as dependent variables, controlling for gender and race.
A final logistic regression model was constructed to examine whether neurocognition and functional status could accurately determine participant group membership at study entry. A binomial logistic regression was performed with neurocognitive domain scores and functional status of both participant groups as independent variables and group as dependent variable, controlling for gender and race.
The logistic regression models contained only significant (p<0.05) domain-specific neurocognitive predictors of social and role functioning. Model calibration was assessed with the Hosmer-Lemeshow (
33) goodness of fit test (p≥0.10). Pseudo R
2 was estimated using the Nagelkerke statistic (an approximate measure of the proportion of explained variation in the logistic model) (
34). The discriminative ability of the regression models was evaluated by the area under the receiver operator characteristic curve (
35) (for more details, see the data supplement that accompanies the online edition of this article).
Results
Table 2 summarizes the baseline demographic and clinical characteristics of the study participants. The healthy comparison and high-risk groups did not differ significantly on age at testing, WRAT-3 reading score, years of education, parental socioeconomic status, handedness, or ethnicity; however, the groups differed significantly on estimated current IQ, race, and gender ratio. The healthy comparison group had a higher mean estimated current IQ and a lower proportion of white males compared with the clinical high-risk group.
The clinical high-risk group demonstrated significant impairments in social (Cohen's d=2.01) and role (Cohen's d=1.78) functioning relative to the healthy comparison group (
Table 2). Social functioning of the clinical high-risk patients ranged from good to an inability to function socially, and role functioning ranged from good to extreme dysfunction. Although significantly correlated with each other, the social and role measures appear to be independent constructs sharing some method variance (healthy comparison group: r=0.37, p<0.001; clinical high-risk group: r=0.29, p<0.001).
At the time of testing, a majority of the clinical high-risk patients (55.9%) were not receiving any medication. The remaining patients (44.1%) were receiving atypical antipsychotics, antidepressants, mood stabilizers, stimulants, and/or anxiolytics. There were no significant effects of medication type or status (medication naive or treated) for clinical high-risk patients on any of the demographic characteristics or neurocognitive domains reported here.
Cognitive Profiles
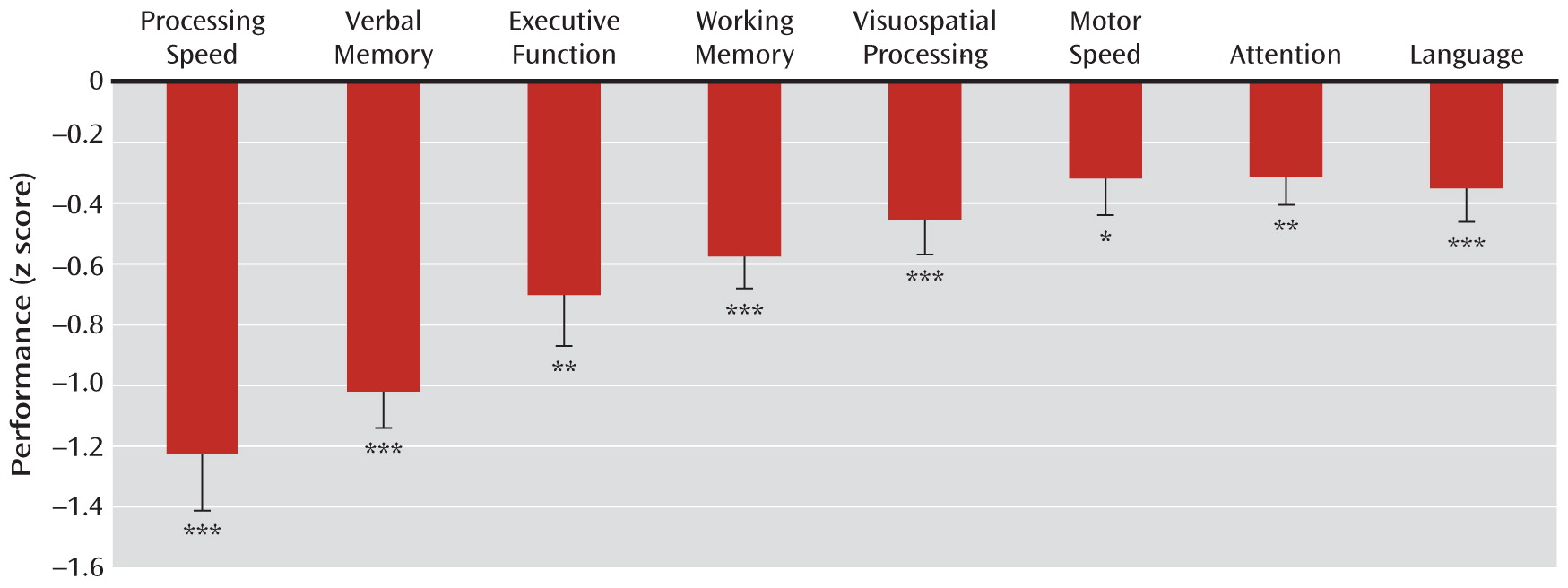
Figure 1 illustrates the mean performance across the eight neurocognitive domains for the clinical high-risk group relative to the healthy comparison group. The results of the MANCOVA indicated a significant main effect for neurocognitive performance (Wilks's lambda=0.88; F=3.73, df=7, 186, p<0.01). Gender was a significant covariate (Wilks's lambda=0.86; F=4.19, df=7, 186, p<0.001), as female patients exhibited poorer global neurocognitive performance than male patients. MANCOVA also revealed that the neurocognitive profile of the clinical high-risk group deviated significantly from flatness, as indicated by the significant interaction between group and neurocognitive performance (Wilks's lambda=0.88; F=3.47, df=7, 186, p<0.01). The clinical high-risk group showed a global neurocognitive impairment (mean=–0.63, SD=0.99) as compared to the healthy comparison group (mean=0.00, SD=0.61). Post hoc paired t tests in the clinical high-risk group indicated that processing speed (t=4.31, df=126, p<0.001) and verbal memory (t=4.91, df=126, p<0.001) were significantly more impaired relative to the mean of all the other neurocognitive domains.
Table 3 summarizes the individual univariate ANCOVAs for each cognitive domain. Individual ANCOVAs revealed that, relative to the healthy comparison group, the clinical high-risk group was significantly impaired in all eight cognitive domains. There was a significant effect of gender on attention (F=6.00, df=1, 193, p<0.05), visuospatial processing (F=22.04, df=1, 203, p<0.001), and language (F=4.89, df=1, 202, p<0.05), as female patients exhibited poorer neurocognitive performance than male patients in these domains.
Relationships Between Cognition and Social and Role Functioning
As shown in
Table 4, global neurocognition was a significant predictor of social and role functioning at baseline. The global neurocomposite score accounted for 8% and 5% of the variance for social and role functioning, respectively. Among the domain-specific neurocognitive scores, processing speed was a significant predictor of social and role functioning at baseline. Processing speed predicted 10% and 7% of the variance for social and role functioning, respectively. This relationship was independent of positive symptoms, as follow-up regression analyses indicated no significant relationships between total attenuated positive symptoms and either social or role functioning.
Given that processing speed was significantly associated with social and role functioning, additional analyses were performed to determine whether processing speed scores could accurately determine functional status when the latter was dichotomized as good or poor functioning. Of the 127 clinical high-risk patients, 83 (65.4%) were in the poor social functioning group and 85 (66.9%) were in the poor role functioning group. Of the 80 healthy comparison subjects, 74 (94.9%) were in the good social functioning group and 73 (93.6%) were in the good role functioning group. The final logistic regression model indicated that participants with higher processing speed scores had a significantly lower likelihood of poor social functioning (odds ratio=0.60, 95% confidence interval [CI]=0.48–0.76; Wald's χ2=18.57, df=1, p<0.001) and poor role functioning (odds ratio=0.62, 95% CI=0.50–0.78; df=1, p<0.001) after controlling for gender and race. The final models accounted for 19% and 18% of the pseudovariance (Nagelkerke's R2) for social and role functioning, respectively. Both models were well calibrated according to the Hosmer-Lemeshow test.
Logistic regression was performed to determine whether neurocognition and functioning could discriminate between clinical high-risk patients and healthy comparison subjects. Processing speed (odds ratio=0.56, 95% CI=0.36–0.87; Wald's χ2=6.72, df=1, p<0.01), social functional status (odds ratio=34.51, 95% CI=8.98–132.70; Wald's χ2=26.56, df=1, p<0.001), and role functional status (odds ratio=20.88, 95% CI=6.58–66.27; Wald's χ2=26.60, df=1, p<0.001) were significant predictors of group membership. The final model was well calibrated and accounted for 71% of the pseudovariance. Participants with impaired processing speed and poor functioning were more likely in the clinical high-risk group.
Discussion
To assess the relationship between cognitive and functional impairments before the onset of psychosis, we examined the baseline neurocognitive performance and functioning of a large group of patients at clinical high risk for developing psychosis. The patients in our study displayed significant neurocognitive impairments, particularly in the domains of processing speed, verbal memory, executive function, and working memory. They also had significant functional impairments, confirming that social and role impairments are present before the onset of psychosis. Our results showed that these functional impairments were related to neurocognitive performance, independent of positive symptoms. Specifically, we found that processing speed was significantly related to social and role functioning, indicating that the relationship between neurocognition and functioning exists before the onset of psychosis and is not an outcome of chronic illness.
Our findings highlight the central role of impaired processing speed in the pathogenesis of schizophrenia and its morbidity. This result is consistent with a number of recent studies that have reported processing speed impairments to be a hallmark feature of the cognitive deficit in patients with schizophrenia (
37) and to be associated with poor functioning (
38). Our results in this study extend this notion to the early stages of the illness and suggest that speed of processing may be particularly important in the development of functional disability. It can be speculated from our findings that processing speed impairments are a rate-limiting factor for developing good social and role functioning during a stage in life when social and occupational skills are rapidly built. Slowing in processing, understanding, and reacting to incoming information is likely to be debilitating in multiple domains of real-world functioning (
37).
In addition to highlighting the impact of cognitive impairments on functioning before the onset of psychosis, these findings provide further support for the core role of cognition in the process leading to psychosis and introduce functional deficits as part of the core disorder. Clinical high-risk patients showed marked neurocognitive impairments across multiple domains, with large impairments seen in processing speed and verbal memory. Impairments in these domains have been well documented in studies of schizophrenia (
2,
39), including in first-episode patients (
25,
40) as well as nonaffected first-degree relatives (
7,
41). Our findings confirm that the cognitive impairment associated with schizophrenia is present and detectable before the onset of psychosis and are consistent with an emerging view that cognition is a critical dimension in individuals at high risk for psychosis.
To our knowledge, this is the first study to examine the relationship between neurocognitive performance and functioning in a large sample of patients at clinical high risk for psychosis and healthy comparison subjects. In a previous study of 45 patients at high risk for psychosis (
18), verbal learning and memory were found to be moderately correlated with social functioning, and reasoning and problem solving were weakly correlated with global functioning. That study, unlike ours, did not find a relationship between functioning and processing speed. Our findings on this relationship may be attributable to our use of the Global Functioning: Social and Role scales to assess functioning; these scales have several advantages over established functional measures currently used throughout the field because they were designed to capture subtle difficulties before the onset of full-blown psychosis, cover the age range typical of the prodromal phase, and disentangle the social and role functioning domains from one another (
17).
Traditionally, prevention of psychosis in clinical high-risk patients has largely focused on the reduction or delay of positive symptoms. A more comprehensive prevention approach should attempt to target the functional impairments that are also present before the onset of psychosis. Therefore, there is a growing need to understand the factors that contribute to poor functioning in individuals at high risk. While impaired cognition serves as an important risk factor, it is not the only explanation for poor functioning. It is possible that other factors, such as independently reduced functional capacity, social stigma, and lack of social support and services, further contribute to the development of functional impairment between adolescence and adulthood. Further research is needed to determine how these factors can be addressed to prevent long-term functional disability.
Acknowledgments
The authors thank the study participants and the staff of the Recognition and Prevention Program for their time and effort from the very outset of these studies. In particular, the authors thank Pradeep Nagachandran, M.D., and Ruth Olsen, B.S., for their assistance in carrying out this study.