Maternal Outcomes
The overall rate of maternal remission during the 12 weeks following treatment initiation was high (67%), and remission rates did not vary by treatment. Relapse rates were low and did not vary by treatment; only seven mothers of 15 children relapsed over the 12 weeks (see Table S2 in the data supplement). Dropout rates were low, with only four mothers and their five children dropping out of the study (see Table S3 in the data supplement).
The results of fitting a mixed-effects regression model with linear and quadratic terms to estimate changes in maternal depression symptoms over time by maternal treatment are presented in Figure S1 in the data supplement. Both linear and quadratic terms were found to be significant but did not vary significantly with treatment. Taken together, the negative linear component (beta1=−2.62, SE=0.17; t=−15.22, p<0.001) and the positive quadratic component (beta2=0.14, SE=0.01; t=9.85, p<0.001) suggest that maternal HAM-D scores decreased significantly and then leveled off for all treatment groups over the 12 weeks.
Child Outcomes
We compared child outcomes over the 12 weeks by maternal treatment, adjusting for child’s age and gender, within-family correlation, and study site. Statistically significant differential treatment effects were observed on CDI and CIS scores. Additional models were tested that included maternal baseline characteristics that were significantly different between treatment groups (age and marital status); these additional variables did not confound results or qualify as moderators and thus were subsequently omitted from the analyses.
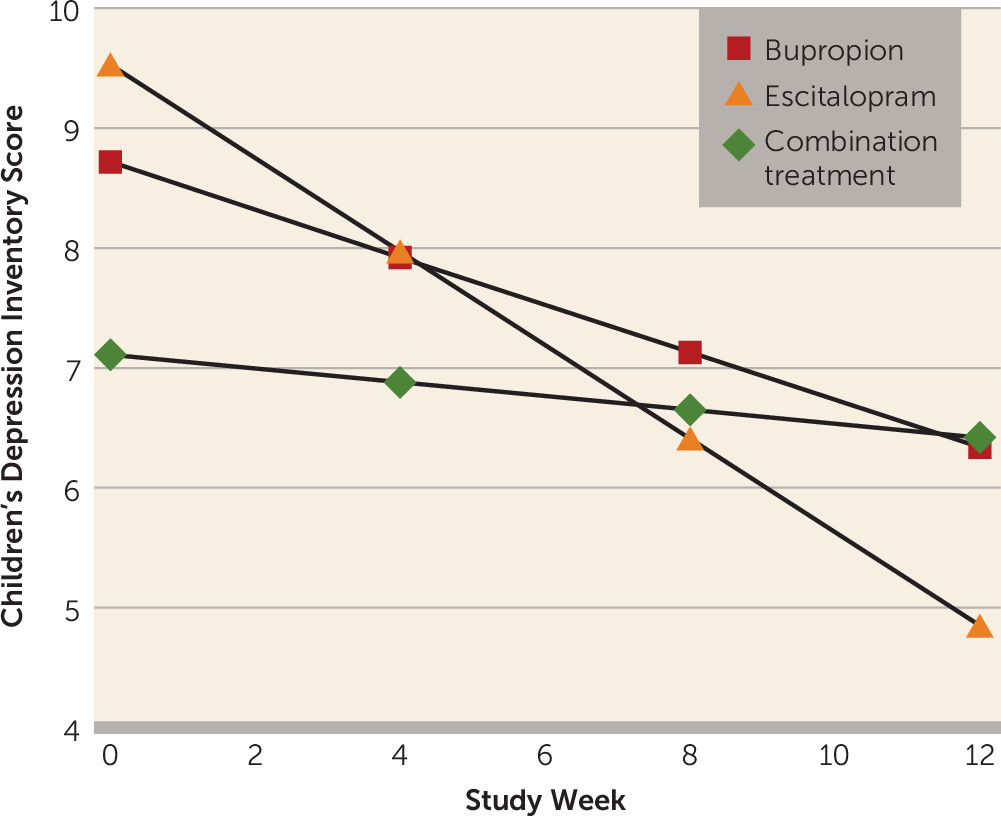
We first determined whether there were significant changes among child outcome measures in each of the maternal treatments separately during the 12 weeks. As shown in
Table 1, mean CDI scores decreased significantly over time among children of mothers who received monotherapy (reflected in the negative beta coefficients and associated p values), indicating that these children became less depressed over time, and those whose mothers received the combination treatment did not. The group-by-time interaction was significant (F=7.28, df=2, 227, p<0.001), suggesting a difference in treatment effect. Post hoc tests revealed that the time trend for children of mothers who received escitalopram monotherapy was statistically different from that of children whose mothers received the combination treatment (t=3.81, p<0.001) or bupropion monotherapy (t=2.04, p=0.04). There were no significant differences between the combination treatment and bupropion monotherapy (
Figure 2).
There was a statistically significant decrease over time in mean CIS scores among children of mothers who received the combination treatment or escitalopram monotherapy (
Table 1), indicating that these children became less impaired over time, as reflected in the statistically significant group-by-time interaction (F=5.57, df=2, 238, p=0.004). Post hoc tests revealed that the time trend for children of mothers who received escitalopram monotherapy was statistically different from that of children whose mothers received the combination treatment (t=2.25, p=0.03) or bupropion monotherapy (t=3.25, p=0.001). There were no significant differences between combination treatment and bupropion monotherapy (see Figure S2 in the online
data supplement).
Changes in Maternal Depressive Symptoms and Treatment Effects on Child Outcomes
Tables 2 and
3 present results of the analysis to determine the relationship between the changes in maternal depressive symptoms over time and the observed effect of treatment on CDI scores. Analyses reported in these tables are based on participants for whom HAM-D data were available.
Table 2 presents models in which the main predictors are maternal treatment assignment, time, and their interaction, and
Table 3 presents models in which mothers’ HAM-D scores were added as a main predictor, along with interactions among HAM-D scores, treatment assignment, and time.
There was a statistically significant interaction between change in maternal depression symptoms over time and treatment (i.e., the week-by-HAM-D score-by-treatment interaction in
Table 3), implying that the association between maternal depressive symptoms and child depressive symptoms over time varied significantly by maternal treatment assignment (p=0.02).
To investigate these results further, we examined each treatment arm separately (
Table 4). This investigation revealed that a reduction in mothers’ HAM-D scores was associated with a reduction in their children’s CDI scores over time, as originally hypothesized, but only for mothers who received escitalopram monotherapy. That is, there was a positive association between HAM-D scores and child CDI scores (beta=0.11, SE=0.05, p=0.03), and the coefficient corresponding to week (number of weeks from baseline) decreased in magnitude and statistical significance with the addition of HAM-D scores as the time-dependent covariate (i.e., the beta coefficient decreased in absolute magnitude from 0.40 [SE=0.06, p<0.001] to 0.27 [SE=0.08, p=0.002]). Furthermore, when the effects of HAM-D scores on child CDI scores were allowed to vary over time (by inclusion of an additional HAM-D score-by-week interaction term) this effect was found to be significant (p=0.001). These results suggest that as maternal HAM-D scores decrease over time, there is a corresponding decrease in child depressive symptoms (CDI scores), but the strength of this association decreased over the first 8 weeks of the study until it leveled off during the last 4 weeks. The effect of HAM-D scores did not vary with time for CDI scores of children whose mothers received bupropion monotherapy (i.e., the week-by-HAM-D score-by-treatment interactions were nonsignificant). Furthermore, there was a negative association between mother’s HAM-D scores and child’s CDI scores on average, and the magnitude of the slope coefficient increased rather than decreased with the addition of mothers’ HAM-D scores as time-dependent covariates, suggesting that a decrease in mother’s HAM-D scores does not explain the decrease in child’s CDI scores for mothers receiving bupropion monotherapy. There was no significant change over time in CDI scores for children whose mothers received the combination treatment, so the question of mediation of change in child CDI scores over time by change in mothers’ depressive symptoms over time became irrelevant for this group.
Similar analyses to determine whether reduction in maternal depressive scores explained the differential treatment effects on child impairment (CIS scores) revealed no significant relationship between improvement in child CIS scores and reduction in mother’s HAM-D scores for any of the three treatments.
Possible Reasons for Maternal Treatment Effect on Children
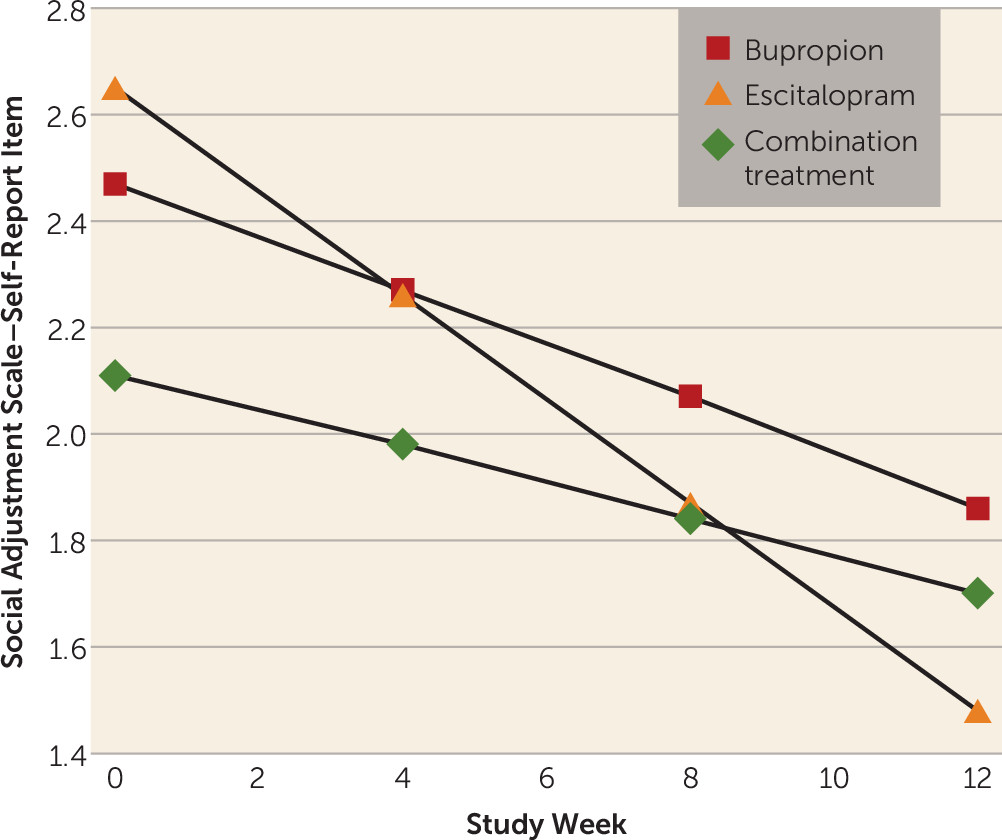
The next exploratory analyses were carried out to examine the effect of maternal treatment on the decrease in children’s depressive symptoms. Results of fitting a regression model to overall maternal social functioning (Social Adjustment Scale–Self-Report) scores showed a significant linear decrease in overall scores over time (beta1=−0.05, SE=0.01; t=−5.84, p<0.001), but the rate of improvement did not vary significantly with treatment. However, when we fitted the same model to the parenting role scores over time, we found a differential treatment effect over time that fell short of statistical significance (p=0.09) (see Figure S3 in the online
data supplement), with mothers receiving escitalopram monotherapy exhibiting greater improvement in parental functioning over the study period as compared with those who received bupropion monotherapy or the combination treatment. This effect was primarily due to a differential effect of escitalopram monotherapy as compared with the other treatments (p=0.02) on mothers’ ratings on the item “being able to talk to and listen to my child” (
Figure 3). The results with parental role scores and the individual parenting items are presented in Table S4 in the
data supplement.
We next examined the child’s report of mother’s care and affection on the Parental Bonding Inventory. The results showed that over time, children of mothers receiving escitalopram monotherapy reported a significant increase in maternal care and affection (beta=0.17, SE=0.08; t=2.00, p=0.047), while those whose mothers received the combination treatment or bupropion monotherapy reported no significant change in maternal care and affection over time. The overall week-by-treatment interaction (F=2.99, df=2, 164, p=0.05) suggested that changes in child-reported maternal care and affection over time differed between treatment groups. This was confirmed in pairwise comparisons, with significant differences observed between children of mothers receiving the combination treatment compared with those whose mothers received escitalopram monotherapy (beta=−0.26, SE=0.12; t=−2.14, p=0.03) and between those whose mothers received bupropion monotherapy compared with those whose mothers received escitalopram monotherapy (beta=−0.26, SE=0.13; t=−2.04, p=0.04), while there were no significant differences between children of mothers who received combination treatment compared with those whose mothers received bupropion monotherapy (see Table S5 in the online data supplement).
There were no significant differential medication side effects or dosing patterns in mothers. The mean daily doses were 23.8 mg for escitalopram monotherapy, 244.8 mg for bupropion monotherapy, and 24.3 mg of escitalopram and 314.3 mg of bupropion for the combination treatment.
There was no significant difference in the percentage of children at baseline or in the past who received psychiatric treatment by maternal treatment. There were, however, significant differences in this measure during the study, with 31.5%, 20.6%, and 8.7% of children whose mothers were receiving the combination treatment, bupropion monotherapy, and escitalopram monotherapy, respectively (p<0.02), receiving psychiatric treatment. These findings suggest that the greater improvement among children whose mothers were receiving escitalopram monotherapy could not be explained by the children having received treatment; the children of mothers in the escitalopram monotherapy group received the least amount of treatment.
Gerra et al. (
25), in a reanalysis of the adult study, examined negative affectivity—which included three domains of aversive moods: guilt, hostility/irritability, and fear/anxiety—as a moderator of treatment response. This approach was based on the suggestion by Nutt et al. (
23) and Stahl et al. (
24) that high negative affectivity is a result of serotonin deficiency and should respond to antidepressants that enhance serotonin neurotransmission—such as escitalopram, as opposed to bupropion. This finding was confirmed in the full adult sample, and we tested it in relationship to the mothers and children. We found that children of mothers with low negative affectivity exhibited a significant decrease in depressive symptoms (CDI scores) over time regardless of mothers’ treatment assignment (see Figure S4 in the online
data supplement). For children of mothers with high negative affectivity, only those whose mothers received escitalopram monotherapy exhibited a decrease in CDI scores (see Figure S5 in the
data supplement).
Further investigation showed that for children of mothers with high baseline negative affectivity, the overall week-by-treatment interaction was significant (F=4.84, df=2, 113, p<0.01), implying that CDI changes over time differed between treatment groups. Individual beta values showed that only children of mothers treated with escitalopram monotherapy (beta=−0.33, SE=0.09, p=0.001) showed a significant reduction in CDI scores over time, whereas children of mothers with high negative affectivity at baseline who received the combination treatment or bupropion monotherapy showed no significant changes over time in CDI scores. However, a formal test of a three-way interaction of differential effects of maternal treatment on children’s CDI scores by maternal baseline negative affectivity did not reach statistical significance, possibly because of lack of statistical power.