Deficits of executive function are present in a wide range of psychiatric disorders, including attention deficit hyperactivity disorder (ADHD) (
1), conduct disorder (
2), and psychotic disorders such as schizophrenia (
3,
4). Executive deficits negatively affect everyday functioning (
5) and contribute to diminished quality of life in many clinical populations (
6,
7). Consequences of executive deficits may be particularly acute in childhood and adolescence, and they may include increased interpersonal conflict, decreased academic achievement, and risk-taking behavior (
7,
8).
Many studies have investigated the neural basis of executive impairments in individual psychiatric disorders. Working memory is one of the most commonly studied components of executive function. For example, meta-analyses in patients with ADHD demonstrate hypoactivation within a network of regions, including the dorsolateral prefrontal cortex, anterior cingulate cortex, thalamus, superior parietal lobule, and precuneus (
9,
10). Patients with schizophrenia also fail to recruit portions of the executive network, such as the dorsolateral prefrontal cortex (
11–
13). Likewise, conduct disorder is associated with a failure to activate a similar network of regions (
14). Taken together, these studies suggest there likely are overlapping effects across disorders, with hypoactivation of the executive system being a common underlying brain phenotype. However, evidence also exists for dissociable abnormalities among psychiatric disorders. For example, hyperactivation of executive regions has been reported in major depressive disorder and anxiety disorders (
15–
17). Nonetheless, some heterogeneity in results exists, and such hyperactivation has been reported in schizophrenia as well (
18).
A case-control design in a single disorder is the predominant approach in the studies reviewed above, and thus most studies are unable to directly evaluate whether common executive deficits exist across disorders. Furthermore, while the incidence of psychiatric comorbidity is quite high, studies typically do not explicitly evaluate its impact. In epidemiological studies, approximately 40% of individuals meeting criteria for one diagnosis met criteria for at least one additional disorder in a different class (
19). Thus, studies that use subjects with “pure” single diagnoses may not be representative. More recently, the neural basis of executive deficits across disorders has begun to be investigated in an attempt to isolate regions of dysfunction specific to each diagnosis (
20). However, studies comparing multiple clinical groups are typically hampered by small sample size, and few prior studies compared more than two disorders at a time. Furthermore, because psychiatric symptomatology exists on a continuum of normal to abnormal, dimensional analyses that cut across categorical clinical diagnoses may both enhance power and improve biological interpretability (
21). Thus, studies in large samples evaluating the effect of multiple dimensions of psychopathology on executive functioning are necessary.
Accordingly, here we used a dimensional approach to examine executive dysfunction with a working memory functional magnetic resonance imaging (fMRI) task in a sample of 1,129 youths imaged as part of the Philadelphia Neurodevelopmental Cohort (
22,
23). Notably, the ascertainment strategy used in the Philadelphia Neurodevelopmental Cohort differs substantially from studies using clinical help-seeking samples, instead employing a community-based approach to examine symptoms in non-help-seeking youths. While such a design will likely have lower symptom severity than clinical samples, a community-based approach may nonetheless be valuable for investigating dimensions of psychopathology. We hypothesized that there would be both common and dissociable deficits in executive recruitment associated with different dimensions of psychopathology. Specifically, we predicted that general psychopathology would be associated with executive hypoactivation regardless of clinical diagnosis. Furthermore, we predicted that specific dimensions of psychopathology would be linked to dissociable regional patterns of impairment within the executive network.
Method
Participants
As has been described, the Philadelphia Neurodevelopmental Cohort is a collaboration between the Center for Applied Genomics at the Children’s Hospital of Philadelphia and the Brain Behavior Laboratory at the University of Pennsylvania (
22,
23). In this report, we consider the entire cross-sectional sample of 1,601 subjects imaged as part of the Philadelphia Neurodevelopmental Cohort; of these, 1,462 completed the
n-back task described below. Following subject exclusions—including medical comorbidity, task nonperformance, and image quality assurance (see the
data supplement that accompanies the online edition of this article)—the final sample included in the analyses comprised 1,129 subjects (mean age=15.5 years, SD=3.4 years; 520 were male). This sample thus constitutes a superset of subjects previously included in reports that focused on effects of working memory performance (
24) and associations with psychosis-spectrum symptoms (
25).
Clinical Assessment
Psychopathology symptoms were evaluated using a structured screening interview (GOASSESS), which has been detailed elsewhere (
26,
27). Computerized algorithms used endorsement of symptoms, their frequency and duration, and the presence of distress or impairment to determine whether DSM-IV criteria were met (see the online
data supplement). The frequency and relevant demographic data for each screening diagnosis considered are detailed in
Table 1. As in large-scale epidemiologic data sets (
19), comorbidity was quite common, with more subjects meeting criteria for more than one category (N=529) than for a single category (N=249).
Psychopathology Factor Analysis
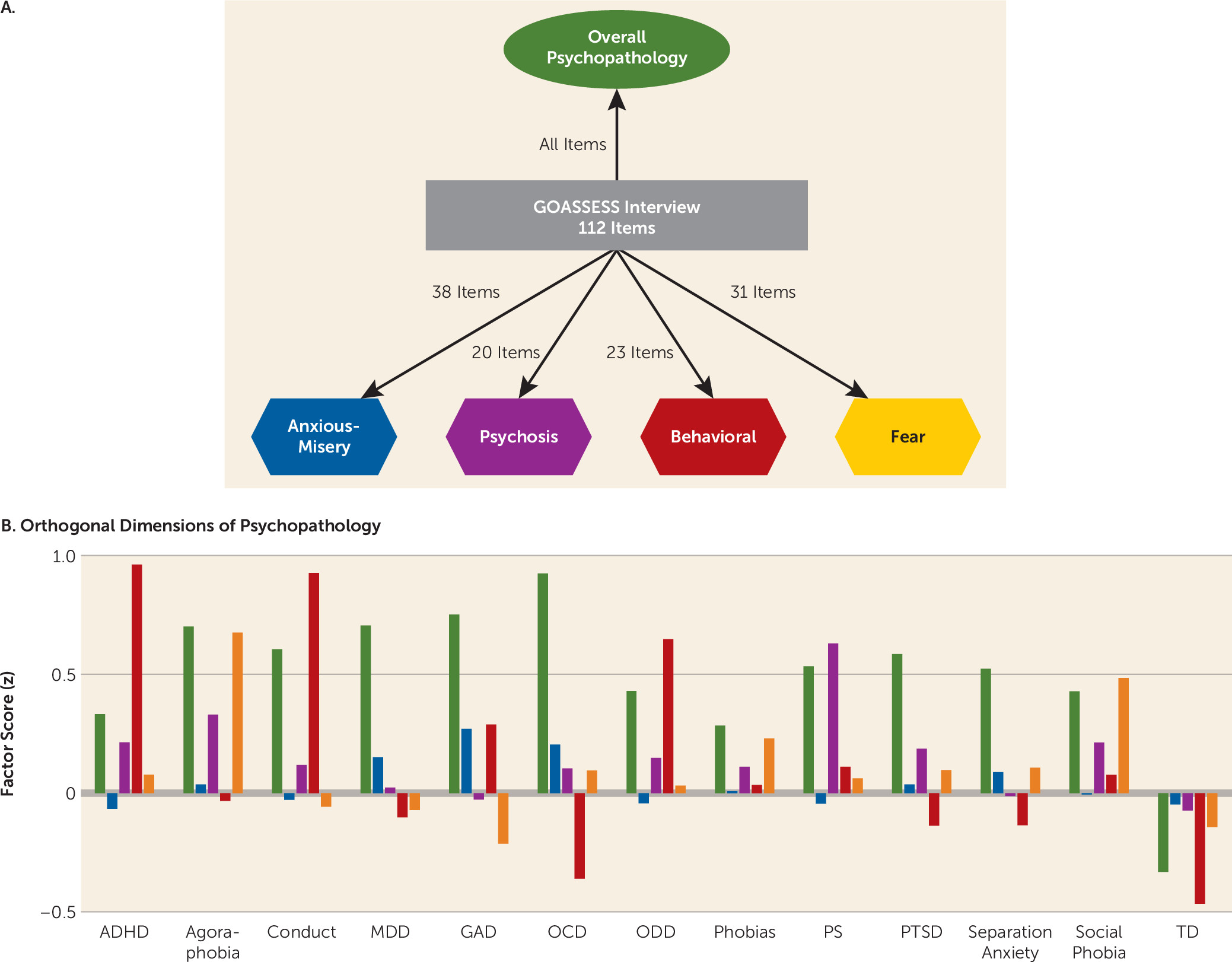
In order to parse this comorbidity into orthogonal dimensions of psychopathology, we performed factor analyses of item-level data from GOASSESS. To produce stable factor scores, analyses included all subjects for whom clinical data were available (N=9,498), rather than only the subset who completed neuroimaging (
26,
27). As initial exploratory factor analyses indicated correlated traits of psychopathology, we used a bifactor confirmatory model, which can produce orthogonal scores from correlated traits (
28).
Methods for estimating the bifactor model will be presented in detail elsewhere (see also the online
data supplement). Briefly, the confirmatory bifactor model (
Figure 1A) was estimated using a Bayesian estimator in Mplus, version 7.1. As predicted by theory and supported by initial exploratory models, the four factors primarily represent anxious-misery (mood and anxiety) symptoms, psychosis-spectrum symptoms, behavioral symptoms (conduct and ADHD), and fear symptoms (phobias). Additionally, the bifactor model estimated a general psychopathology factor, representing the overall burden of psychopathology while controlling for the presence of specific symptom dimensions. Importantly, all five factors (including general psychopathology) from the bifactor model are orthogonal and can be considered jointly in analysis of imaging data. Factor scores within each of the categorical screening categories were as expected, given item-level loading (
Figure 1B).
Task Paradigm, Image Acquisition, and Image Processing
Task paradigm, image acquisition, and preprocessing methods were as previously reported (
24). Briefly, a fractal version of the
n-back task (
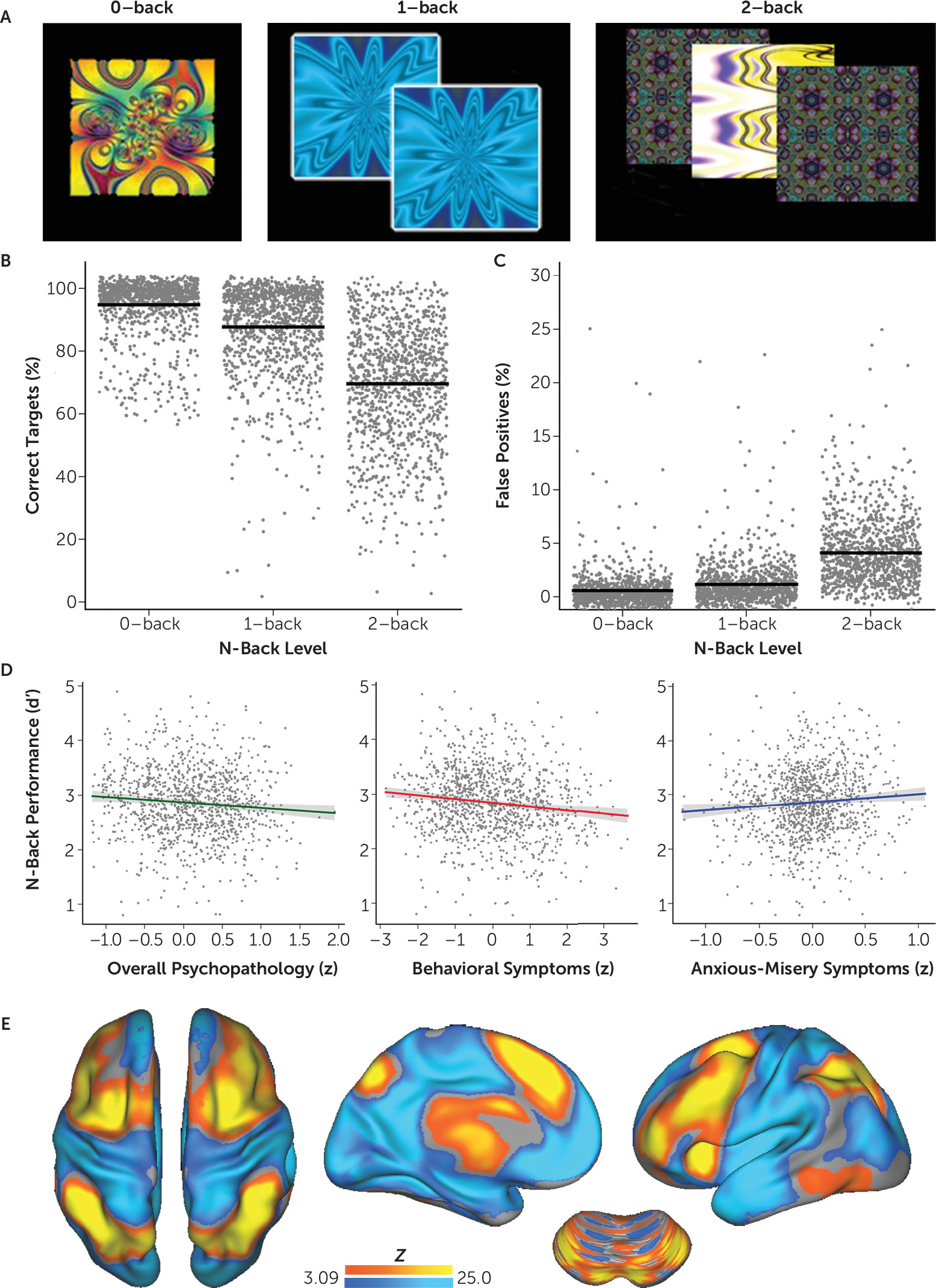
29) was used to probe executive system function across three levels of working memory load (
Figure 2A). The primary behavioral measure was
d′, a signal detection metric that limits the influence of response bias. Task performance (d′) across all levels of working memory load was related to categorical diagnosis from GOASSESS and dimensional factor scores using linear models while controlling for age and sex. The Bonferroni correction was used to account for the testing of five dimensions of psychopathology.
A single scanner (Siemens 3-T Tim Trio) was used to acquire fMRI, T
1, and B
0 images for all subjects (see the
data supplement). Time-series analysis of subject-level imaging data used the FMRIB Software Library version 5.0 (
http://www.fmrib.ox.ac.uk/fsl) (FSL) (
30) to model three condition blocks (0-back, 1-back, and 2-back); the primary contrast was 2-back > 0-back, which robustly recruits the executive network (
24). Subject-level statistical maps were distortion corrected, coregistered to the T
1 image using boundary-based registration, normalized to the Montreal Neurological Institute’s (MNI) 152 1 mm template using Advanced Normalization Tools (
31), and then downsampled to 2 mm. All transformations were concatenated so only one interpolation was performed.
Multivariate Group-Level Analysis: Global Associations With Psychopathology
As a first step, we evaluated the degree to which dimensions of psychopathology from the factor analysis affected overall multivariate patterns of activation (see Figure S1 in the online
data supplement). To do this, we used multivariate distance-based matrix regression, a statistical technique developed originally for large-scale ecologic data sets but which has recently been used in image analysis (
32); this was implemented using the “vegan” package in R (
33). Subject-level activation maps are compared on a pairwise basis (Euclidean distance) to yield a distance matrix. Matrix regression is then used to test whether each phenotypic variable explains the distances among each participant’s activation patterns. In contrast to other multivariate methods, this approach allowed us to examine the influence of multiple dimensions of psychopathology simultaneously while also controlling for covariates (age, sex, and in-scanner motion). Multiple tests were accounted for using the Bonferroni correction as above.
Mass-Univariate Group-Level Analysis: Regional Associations With Psychopathology
While the multivariate analysis detailed above provided an estimate of the degree to which dimensions of psychopathology affected the global pattern of activation, this analysis does not evaluate regional effects. Accordingly, we next conducted a standard mass-univariate analysis using a general linear model implemented in FSL (
30). We evaluated the effect of each dimension of psychopathology within this linear model, with covariates as noted above. Additionally, we investigated whether dimensions of psychopathology that showed a significant effect were significantly different from one another using an F test across dimensions. Type I error control was provided by cluster correction using 10,000 Monte Carlo simulations (voxel height of z>3.09; cluster probability of p<0.001; minimum cluster size of k=67) (
34). All analyses described below used unmasked, whole-brain, voxelwise data. Images were displayed using Caret.
Supplementary Analyses
To evaluate whether potentially confounding factors influenced observed results, we conducted a series of supplementary analyses. These included removing the minority (11.4%) of subjects who were taking psychoactive medication; counting task performance (d′) as a covariate; excluding subjects with poor performance (>7 nonresponses) on the 2-back condition; and using mean accuracy on an out-of-scanner computerized neuropsychological battery, race, assessment-scan interval, maternal education, and in-scanner performance (d′) together as covariates in one model.
Results
Multiple Dimensions of Psychopathology Affect Task Performance
As expected, increasing working memory load was associated with fewer correct responses to targets and increased false positive responses to foils (
Figure 2B and
Figure 2C). These measures were integrated using the signal detection measure d′. As expected, d′ varied considerably by screening categorical diagnosis (see
Table 1). When summarized as psychopathology dimensions, several factors significantly affected working memory task performance (
Figure 2D). Higher levels of both overall psychopathology (t=2.58, df=1124, p=0.001) and behavioral symptoms (t=3.82, df=1124, p=0.0001) were associated with lower working memory performance. In contrast, higher levels of anxious-misery symptoms were associated with a trend toward better working memory performance that did not survive correction for multiple comparisons (t=2.45, df=1124, p=0.015). There was not a significant relationship between fear or psychosis and working memory performance.
Overall Psychopathology Alters Global Patterns of Executive System Recruitment
As expected, the 2-back > 0-back contrast robustly recruited the entire executive network and resulted in deactivation of nonexecutive regions (
Figure 2E). We next evaluated whether dimensional psychopathology was associated with changes in this multivariate pattern of activation and deactivation using multivariate distance-based matrix regression. This procedure revealed that overall psychopathology was associated with a significant disturbance in the global pattern of executive system recruitment (p=0.009). Other symptom dimensions did not have a significant relationship; nonsignificant trends were observed for behavioral (p=0.044) and anxious-misery (p=0.092) symptoms.
Dimensions of Psychopathology Differentially Affect Regional Executive Activation
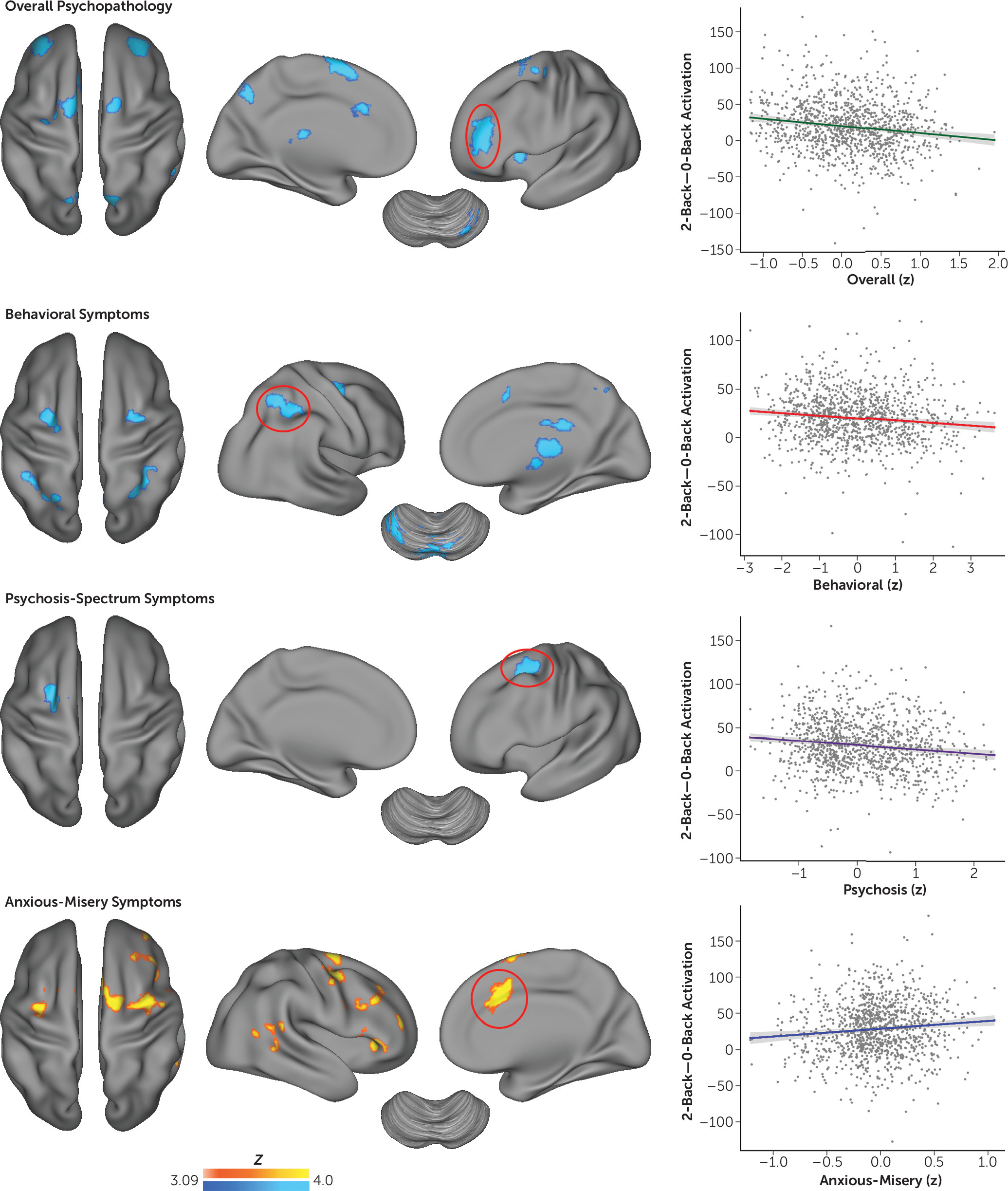
Multivariate distance-based matrix regression evaluates the presence of global multivariate effects, but it is not sensitive to regional changes. Accordingly, we used mass-univariate generalized linear models to examine the relationship between dimensions of psychopathology and executive system activation (
Figure 3; see also Table S1 in the online
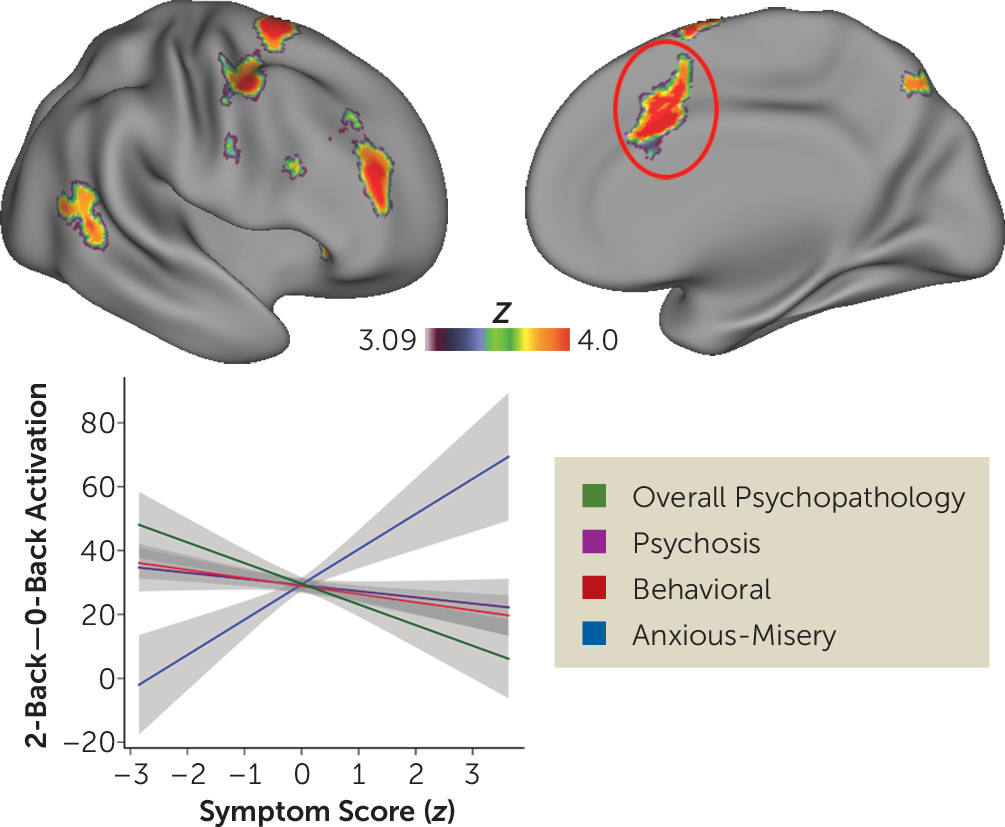
data supplement). As suggested by multivariate results, overall psychopathology had the most robust impact on executive activation. Higher levels of overall psychopathology were associated with diminished activation of the left and right frontal pole, anterior cingulate cortex, anterior insula, thalamus, and precuneus. Behavioral symptoms were associated with diminished activation of the frontoparietal cortex as well as the thalamus and cerebellum. Psychosis-spectrum symptoms were associated with diminished activation of the left dorsolateral prefrontal cortex. Fear (phobia symptoms) was associated with diminished activation of a marginally significant cluster of the medial frontal cortex, but this effect did not survive supplementary analyses (see below) and was not evaluated further. Finally, in contrast to the hypoactivation described above, anxious-misery symptoms were associated with a marked hyperactivation of multiple executive regions including the anterior cingulate cortex, dorsolateral prefrontal cortex, parietal cortex, and thalamus. Notably, a small area of overlapping significant effects across dimensions was seen in the left dorsolateral prefrontal cortex (MNI coordinates: x=−28, y=−6, z=58; k=10). Significant differential effects of each dimension were present in multiple executive regions (
Figure 4; see also Table S2 in the
data supplement). These effects were driven by the divergent impact of global psychopathology (hypoactivation) and anxious-misery (hyperactivation). We did not find any differences in default mode regions where task-induced deactivation was present.
Supplementary Analyses
Overall, convergent results were obtained from supplementary analyses when participants taking psychoactive medications were excluded (Table S3 in the data supplement), when working memory performance was included as a covariate (Table S4 in the data supplement), and when multiple additional covariates were included (Table S5 in the data supplement). When participants with poor performance on the 2-back condition were excluded, psychosis-spectrum symptoms were associated with a marginally significant cluster of hyperactivation in the cingulate gyrus; otherwise, results were similar (Table S6 in the data supplement). As noted above, fear did not have a significant association with activation in any of the supplementary analyses.
Discussion
In this large study of psychopathology in youth, we examined associations between diverse psychopathology and activation of the brain’s executive system during a working memory task. To account for the fact that psychiatric disorders are highly comorbid, factor analysis of the psychopathology data using a bifactor model allowed us to examine both general psychopathology and orthogonal dimensions of psychopathology. Overall psychopathology was associated with a significant alteration of the global multivariate pattern of activation. Furthermore, dimensions of psychopathology significantly influenced regional patterns of activation in different ways. In contrast to the hypoactivation seen in association with overall psychopathology, anxious-misery symptoms were associated with hyperactivation of the same network. Taken together, these data emphasize that dimensions of psychiatric symptomatology are associated with both common and distinct deficits within the executive system.
Evidence for Common Executive Deficits Across Psychiatric Syndromes in Youth
The most robust finding in the present study is evidence of common executive deficits attributable to overall psychopathology present across categorical clinical diagnoses. This was observed on both a global and local scale: overall psychopathology was associated with an alteration of the global multivariate pattern of activation, driven by regional hypoactivation in a network of executive regions including the frontal pole, anterior cingulate, anterior insula, and precuneus. These results accord with a copious literature of case-control studies from multiple individual disorders and, moreover, highlight the centrality of executive dysfunction across major psychiatric syndromes.
We were able to estimate the influence of overall psychopathology across disorders through the use of a bifactor analysis of the item-level responses from the psychopathology screening interview. This approach obviates several major obstacles to estimating common deficits across traditional categorical diagnoses. First, when comorbidity is represented in standard models as shared variance, it is controlled for but cannot be estimated. Estimating overall psychopathology may be particularly important in youths, where the pattern of psychopathology may not fit standard diagnostic criteria in the context of ongoing development. Second, strongly nonrandom patterns of comorbidity exist between different disorders (
19). For example, mood and anxiety disorders have a high rate of comorbidity, as do ADHD and conduct disorder. Third, the frequency of each diagnosis varies considerably; thus, the statistical power to estimate the effect of each individual diagnosis is not equal across effects. Fourth, categorical diagnoses are by definition dichotomous, and important dimensional effects cannot be examined.
Crucially, each of these problems can be overcome through modeling of latent dimensions of psychopathology. Here we used confirmatory factor analysis to estimate a bifactor model, which provides orthogonal scores for every subject in each psychopathology domain, as well as a score for general psychopathology. These orthogonal scores can thus be included in a single model that can estimate dissociable dimensions of psychopathology with equal power, as well as the impact of overall psychopathology through the general score. The regions implicated by overall psychopathology include the frontal pole, anterior cingulate cortex, and anterior insula. Notably, all these regions are part of the cingulo-opercular control network (also called the salience or ventral attention network), which is consistently identified as among the most reproducible large-scale functional brain networks both at rest and during task performance (
35). This network is critical for cognitive control and is particularly implicated in maintenance of task sets and error monitoring (
36). These regions are among the most commonly impaired in case-control studies of schizophrenia, ADHD, and conduct disorder, suggesting that dysfunction of this network is not associated with one specific domain of psychopathology (
4,
10,
37). Further support of this theory is provided by results of a large-scale meta-analysis by Goodkind et al. (
38), who found that gray matter loss in these cingulo-opercular regions was present across psychiatric disorders and was associated with impaired executive function.
Orthogonal Dimensions of Psychopathology Are Associated With Dissociable Deficits
In addition to robust effects of overall psychopathology, we also identified both regionally and directionally dissociable effects of specific dimensions of psychopathology. Behavioral symptoms were associated with diminished activation of a network of regions, including the frontoparietal cortex, thalamus, and cerebellum, whereas psychosis-spectrum symptoms were associated with hypoactivation of the left dorsolateral prefrontal cortex. The behavioral dimension loaded most prominently onto items assessing externalizing disorders such as ADHD, conduct disorder, and oppositional defiant disorder. The results are consistent with case-control studies in each of these disorders, which have demonstrated hypofunction of executive regions including, most commonly, frontoparietal regions (
10,
20). Similarly, dysfunction of the dorsolateral prefrontal cortex is considered one of the cardinal impairments of psychosis (
39). However, it should be noted that the effects of the psychosis spectrum observed here are more circumscribed than those typically reported; this may be due to the use of a population rather than a clinical sampling strategy, a focus on subthreshold psychosis symptoms, and accounting for overall psychopathology through the bifactor model. However, consistent with the present results, our previous report on psychosis-spectrum symptoms in this sample observed that hypoactivation of executive regions was linked to cognitive performance but not to severity of positive psychotic symptoms, suggesting a substantial effect of overall impairment rather than psychosis per se (
25).
In contrast to the diminished recruitment seen in other symptom domains, anxious-misery symptoms were associated with widespread hyperactivation of the executive network. Indeed, after accounting for overall psychopathology, anxious-misery symptoms were associated with a trend toward better working memory performance. However, executive hyperactivation is unlikely to be simply an epiphenomenon associated with working memory performance, as hyperactivation was still seen in sensitivity analyses that controlled for both in-scanner and out-of-scanner cognitive performance. This result is consistent with several studies of executive function in major depression and anxiety disorders that have found overactivation of executive regions (
15–
17). In contrast to the deficient ability to recruit the executive network seen in association with other symptom dimensions, these data suggest that mood and anxiety symptoms are associated with an inefficient executive system, where higher levels of executive network activation occur. It should be noted that participants in our sample who screened positive for mood and anxiety disorders frequently also had high levels of overall psychopathology. As these dimensions have directionally opposite associations with executive recruitment, the relative presence of comorbidity in a given sample could conceivably result in divergent results.
Limitations
Certain limitations of the present work should be noted. First, the young community-based sample studied here had diminished symptom severity compared with that found in typically older help-seeking samples drawn from clinical practices. Drawing from a different distribution of symptoms may result in reduced generalizability to clinical populations. However, the approach used here allowed us to accrue a larger, mainly unmedicated sample at a single site and scanner, and this approach is well suited to investigating broad dimensions of psychopathology. A second important limitation is that this cross-sectional report does not include longitudinal data. Our prior work in this data set has demonstrated that between-subject cross-sectional age effects are relatively subtle (
24); within-subject longitudinal data will be particularly informative regarding whether specific symptom domains are associated with the development of executive deficits over time. Third, certain important classes of psychopathology, such as substance use, that have been shown to affect executive function were not considered in the present analysis (
40). Fourth, despite prior research showing abnormalities of the default mode network in multiple psychiatric disorders, we did not find significant effects in the default mode network; this may be due to the task paradigm employed. Finally, as previously noted (
26,
27), the highly structured screening format of GOASSESS may have resulted in high sensitivity but relatively diminished specificity, potentially leading to overestimation of the frequency of several disorders. Alternatively, high rates of endorsed symptoms may accurately reflect this particular sample. Although factor-analytic results of GOASSESS appear consistent with prior findings and lend support to the validity of the measure, ongoing longitudinal follow-up with semistructured assessments applying DSM criteria will allow further evaluation of the clinical relevance and stability of the present findings.
Conclusions
These data provide novel evidence regarding common and dissociable executive system deficits across multiple dimensions of psychopathology in young people. The results emphasize that executive dysfunction is present in association with overall psychopathology across traditional categorical psychiatric diagnoses, underscoring this system’s central relevance for circuit-based conceptualizations of neuropsychiatric disorders such as the National Institute of Mental Health’s Research Domain Criteria (
21). These results may suggest that interventions seeking to enhance executive function may not fit well within the existing categorical diagnostic framework and may be beneficial to individuals across diverse clinical syndromes where executive deficits are present. Future research employing longitudinal designs may motivate targeted early interventions that seek to mitigate executive dysfunction in youths before negative outcomes accrue.
Acknowledgments
The authors thank the acquisition and recruitment team: Karthik Prabhakaran, Jeff Valdez, Raphael Gerraty, Marisa Riley, Jack Keefe, Elliott Yodh, Nicholas DeLeo, and Rosetta Chiavacci. The authors also thank Frank Mentch for data management.