Irritability can be defined as an increased propensity to experience anger and frustration, compared with peers (
1). It is a serious and common mental health problem among youths (
1,
2). It predicts adult depressive and anxiety disorders (a genetically mediated association) as well as long-term impairment (e.g., high suicidality and low educational and income attainment) (
1–
3). Irritability is also a core feature of the new DSM-5 category of disruptive mood dysregulation disorder, which is characterized by developmentally inappropriate, frequent, and severe temper outbursts (
2,
4). Youths with severe irritability experience significant impairment in multiple domains (e.g., at home, in school, and with peers) and have high rates of service use, hospitalization, and school suspensions (
2,
4). However, there are few evidence-based treatments for irritability (
5). A better understanding of the pathophysiology of irritability is essential to guide the development of novel mechanism-based treatments for this common and impairing problem.
Given the increased proneness to frustration associated with irritability, and working from a translational neuroscience perspective, the neural mechanisms mediating irritability can be captured by studying an organism’s neural responses to frustrative nonreward (
1,
2). Originally operationalized in rodents, frustrative nonreward is the psychological state induced by the failure to receive a reward that an organism has been conditioned to expect (
6). In humans, investigators can model frustrative nonreward during functional MRI (fMRI) by evoking frustration in real time while assessing its neural correlates (
2). In a large, transdiagnostic sample, we used such methods to examine associations between irritability and neural activation during an fMRI paradigm that models frustrative nonreward.
Our second goal was to examine age-related variation in the association between irritability and neural activity. Normative responses to frustration change during development (
7), yet little research has examined the underlying neural mechanisms (
8). Indeed, while many behavioral studies document the development of emotion regulation (
9,
10), neuroimaging research has just begun to elucidate the maturation of brain systems supporting such capacity (
11,
12). For example, the protracted development of the prefrontal cortex has been linked to age-related improvements in attention shifting and cognitive control, which can modulate affective arousal (
11–
14). Although age-related brain mechanisms have been examined in some clinical populations (e.g., in persons with anxiety or depression) (
15,
16), little work has been done on irritability (
8,
17,
18).
Few studies utilize frustration paradigms in youths with irritability, and those that do report neural dysfunction in the prefrontal cortex and anterior cingulate cortex, striatum, amygdala, and parietal cortex (
19–
22). However, the sample sizes of these studies are insufficiently large to generate clear conclusions (
23). To address this issue, we recruited a relatively large sample of 195 youths. Moreover, no previous fMRI research, to our knowledge, has dissociated neural responses to a frustrating event from the impact of frustration on the neural mechanisms mediating performance on a subsequent cognitive trial. Here, we examined the neural response to frustration and the impact of frustration on attention orienting. We defined the latter as the ability to disengage attention from the current focus, move attention to a selected alternative target, and direct attention to that target (
24). We chose an attention-orienting task that engages frontal systems (e.g., the ventral attention network) known to mediate this process (
14) and adapted a frustrating task used previously (
19) to track adjustments in this brain system following frustration. The task induces frustration by falsely informing study subjects that they have committed errors. Given the critical role of the executive attention system in adapting to errors (
14), this system may thus be important in the context of frustration. It is also noteworthy that recent work has implicated attention orienting (in response to threat) as a potential mechanism of irritability (
1,
2,
25).
We examined associations between irritability, measured dimensionally, and neural activations during a frustrating attention-orienting task. Specifically, we studied a transdiagnostic clinical sample of 195 youths with varying levels of irritability. Based on previous work (
19–
22), we hypothesized that irritability would be associated with perturbed activation in frontal-striatal-amygdalar regions during feedback processing and during attention orienting following frustrating feedback. Additionally, given the protracted nature of prefrontal cortex development (
12,
13), we hypothesized that associations between irritability and activation in this region would be moderated by age.
Method
Participants
The study sample included 195 children and adolescents ages 8–18 (mean age, 12.9 years [SD=2.3]) with well-distributed irritability levels and a mean above the cutoff for severe irritability (
26). Participants had primary diagnoses of disruptive mood dysregulation disorder (characterized by severe, chronic irritability) (N=52), anxiety disorder (N=42), attention deficit hyperactivity disorder (ADHD) (N=40), or no disorder (N=61) (
Table 1) (for further details, see Tables S1 and S2 in the
online supplement). They were recruited from National Institute of Mental Health (NIMH) clinics between February 2012 and July 2016. On the Children’s Global Assessment Scale (
27), 58% of the sample had a score ≤60, indicating at least “some noticeable problems” in several areas. Most were seeking or receiving treatment; 48% were being treated with medication, and half of these were taking two or more types of medications. Some patients with disruptive mood dysregulation disorder were in an inpatient treatment trial. Patients with anxiety disorder were in an outpatient treatment trial. This study was approved by the NIMH institutional review board, and written consent and assent, respectively, were obtained from parents and children. Exclusion criteria included an IQ <70, pervasive developmental or neurological disorders, substance abuse within the past 2 months, and lifetime history of psychosis, conduct disorder, or unstable or chronic medical illness. For detailed diagnostic and clinical assessments and additional inclusion and exclusion criteria for the anxiety disorder group, see the Methods section in the
online supplement.
Measures
We assessed irritability using parent and child reports from the Affective Reactivity Index (ARI) (
28). To control for co-occurring anxiety and ADHD symptoms, we collected parent and child reports from the Screen for Child Anxiety Related Emotional Disorders (SCARED) rating scale (
29) and parent reports from the Conners’ Parent Rating Scale–Revised (CPRS-R) long form (30). Total scores from child and parent reports were averaged for the ARI and SCARED. The ADHD index T score from the CPRS-R was used to index ADHD symptoms. For participant distribution (by diagnosis) along these three symptom dimensions, see Figure S1 in the
online supplement. For more than 90% of the sample, these measures were collected within 3 months of scanning. The 3-month window was selected on the basis of the stability of the measures and to maximize the sample size. These measures have been shown to be highly stable across periods longer than 3 months (e.g., see Stringaris et al. [
28]; see also the Methods section in the
online supplement).
fMRI Paradigm
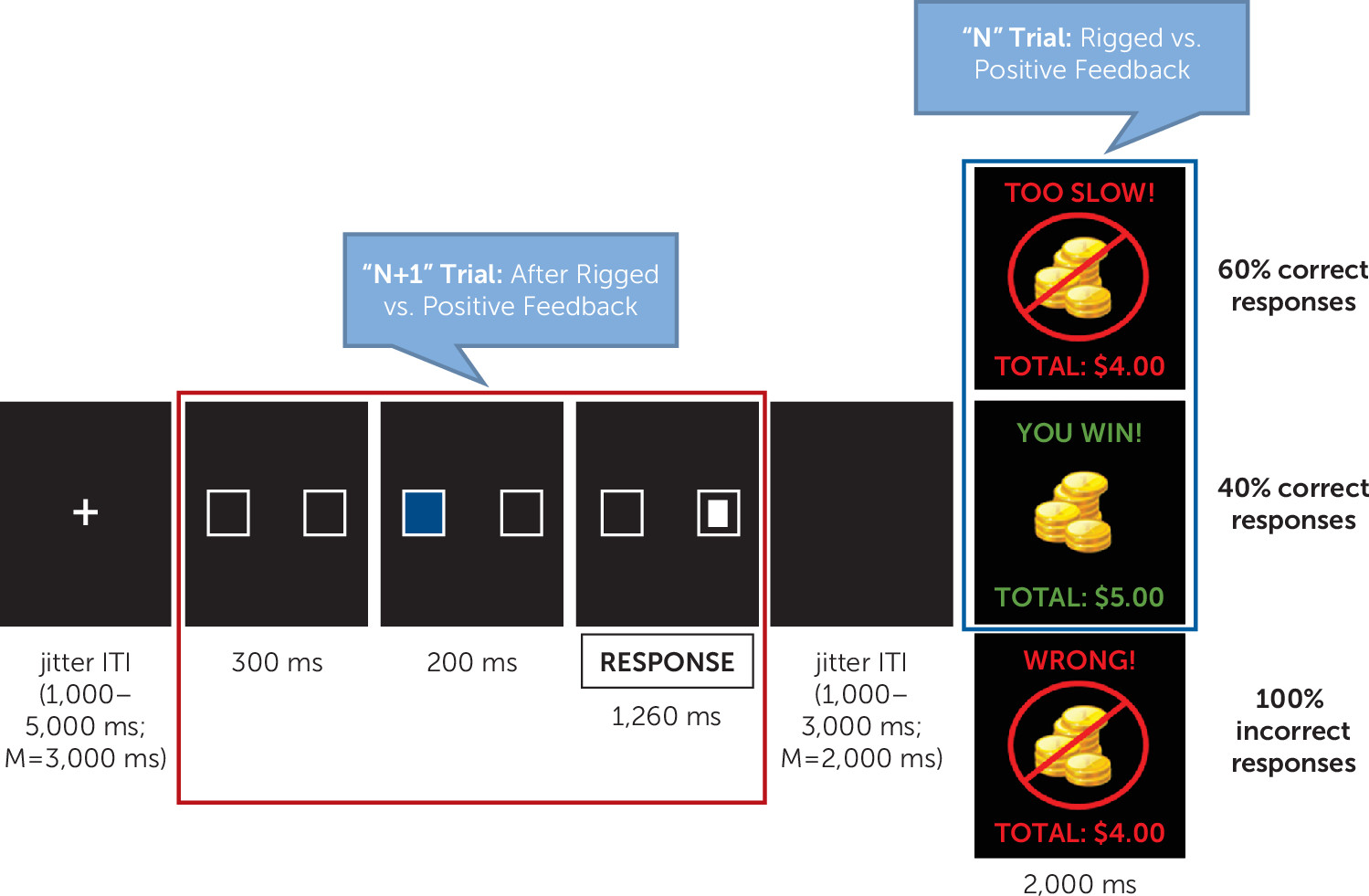
Participants completed a frustrating attention task, the affective Posner 2 paradigm (
31), which was adapted from a previous fMRI study (
19) and demonstrates good reliability and validity (
31). Participants were asked to identify a target following a cue by button press (left or right). The target appeared in the same location as the cue (valid trials) for 75% of trials and in the opposite location (invalid trials) for 25%. The task consisted of two non-frustration runs during which participants received accurate or positive feedback and two frustration runs during which they received rigged or positive feedback (60% and 40% of correct trials, respectively). Each run lasted 8 minutes (
Figure 1) (see also Figure S2 in the
online supplement). Imaging analyses focused on the two frustration runs. Group-level analyses were conducted separately for the feedback and attention portions of the task, which were separated by jitter (1,000–3,000 ms, with an average of 2,000 ms) (
Figure 1). The feedback portion (
Figure 1) probed neural activity during processing of rigged feedback compared with positive feedback. The attention portion (“N+1” trial) (
Figure 1) assessed neural activity during the attentional event following rigged feedback compared with positive feedback. At the end of each run, participants rated their feelings of unhappiness and frustration on 9-point Likert scales. For further details regarding task procedures, see the Methods section in the
online supplement.
Imaging Acquisition
Neuroimaging data were acquired on a 3-T General Electric scanner (General Electric, Boston) using an eight-channel head coil. A high-resolution anatomical scan (1-mm slices, three-dimensional spoiled gradient-echo sequence, 7° flip angle, minimum full echo time, 256×256 matrix, 25.6-cm field of view) and gradient echo-planar imaging images were collected (repetition time=2,300 ms, echo time=25 ms, 24-cm field of view, voxel size=2.5×2.5×3 mm, 206 volumes per run, flip angle=75° [N=134] or 90° [N=61]). For further details, see the Methods section in the online supplement.
Imaging Preprocessing and Individual Analysis
Data were analyzed using Analysis of Functional NeuroImages (AFNI). Preprocessing included despiking, temporal alignment to the first acquired slice, coregistration, smoothing, masking, and intensity scaling. Repetition time pairs with a Euclidean norm motion derivative >1 mm were censored during linear regression. A general linear model estimated voxel-wise blood-oxygen-level-dependent signal change (see the Methods section in the online supplement).
Data analyses.
Behavioral and post hoc imaging analyses were conducted with SPSS (IBM, Armonk, N.Y.). For behavioral results (frustration and unhappiness ratings, accuracy, and reaction time), see the Results section in the
online supplement. Group-level whole-brain activation analyses were conducted using AFNI’s 3dMVM, a multivariate model-based analysis of covariance (ANCOVA) appropriate for our fMRI data and study design (
32). Analyses examined the effects of ARI, age, and the ARI-by-age interaction, with SCARED score, CPRS-R score, and motion (for imaging data) as covariates. All variables were continuous, and they were mean centered to reduce multicollinearity due to testing of the interaction term (ARI by age). Correlations between dimensional measures (rs <0.54) had an acceptable tolerance in the model with ARI, age, ARI-by-age interaction, SCARED score, CPRS-R score, and motion (variance inflation factors ≤1.46) (for further details, see Table S2 in the
online supplement).
Imaging data.
Imaging analyses focused on frustration runs because only these runs contained both positive and rigged feedback. We did not directly compare non-frustration and frustration runs (e.g., on positive feedback) because there was a fundamental difference in “baselines”: the baselines in frustration runs were likely to be more saturated and elevated. However, we conducted a whole-brain analysis for non-frustration runs only and, compared with the baseline, found no significant associations between irritability and neural activation during either positive feedback or attention orienting following positive feedback.
Only valid, correct trials were included, given the insufficient numbers of other trial types. Separate analyses were conducted for the feedback portion of the N trial and the attentional portion of the N+1 trial (
Figure 1). For the feedback portion of the N trial, an ARI-by-age-by-condition (rigged compared with positive feedback) ANCOVA was conducted to assess activation during processing of rigged feedback compared with positive feedback and how it varied with irritability and age. For the N+1 trial, an ARI-by-age-by-condition (after rigged compared with positive feedback) ANCOVA was conducted to assess activation during the attentional event following rigged feedback compared with positive feedback and how it varied with irritability and age.
A whole-brain gray matter mask was used in the analyses, including voxels for which data existed for ≥90% of participants, voxel-wise p values of 0.001, and multiple-testing correction alphas of 0.05 via Monte Carlo cluster-size simulation. By using methods designed recently to address concerns regarding inflated false positive rates (
33), the cluster size surviving whole-brain correction was set at 703 mm
3. At this threshold, we observed a large cluster of 68,000 mm
3 for the three-way ARI-by-age-by-condition interaction and a cluster of 86,281 mm
3 for the two-way ARI-by-condition interaction from the N+1 analyses. To facilitate interpretation, we extracted clusters by using a more stringent voxel-wise p value of 0.0001 (clusters ≥203 mm
3) (
Table 2). Using this threshold, we created a conjunction map of the significant three-way and two-way interactions and calculated the shared voxels (i.e., voxels that showed significant effects for both the three-way ARI-by-age-by-condition interaction and the two-way ARI-by-condition interaction). Only 8.4% of the voxels were shared, suggesting that most voxels that showed a significant ARI-by-condition interaction were not moderated by age. To deconstruct significant interactions, mean activation across voxels in significant clusters were extracted using AFNI’s 3dROIstat for follow-up analyses in SPSS. For the N trial analyses, no significant clusters were observed (i.e., ARI, age, and their interaction were not associated with activation during processing of rigged feedback compared with positive feedback).
To address concerns regarding threshold-based cluster forming and replicability of imaging findings, we reanalyzed our data using the threshold-free cluster enhancement approach (
34), with a family-wise error rate correction level of 0.05, by permutation testing (for further details, see the Results section and Figure S9 in the
online supplement). Results were largely consistent with the original analysis in AFNI, except that two small clusters for the ARI-by-condition effect (in the left caudate and precentral gyrus) became nonsignificant (the right caudate remained significant).
Results
Imaging Data
Significant findings emerged from the N+1 analysis, in the comparison of neural activity occurring after rigged feedback compared with positive feedback during the frustration runs (
Figure 1). Specifically, we report interactions involving condition (after rigged compared with positive feedback) and ARI (dimensional measure of irritability) (i.e., ARI-by-age-by-condition and ARI-by-condition).
ARI-by-age-by-condition.
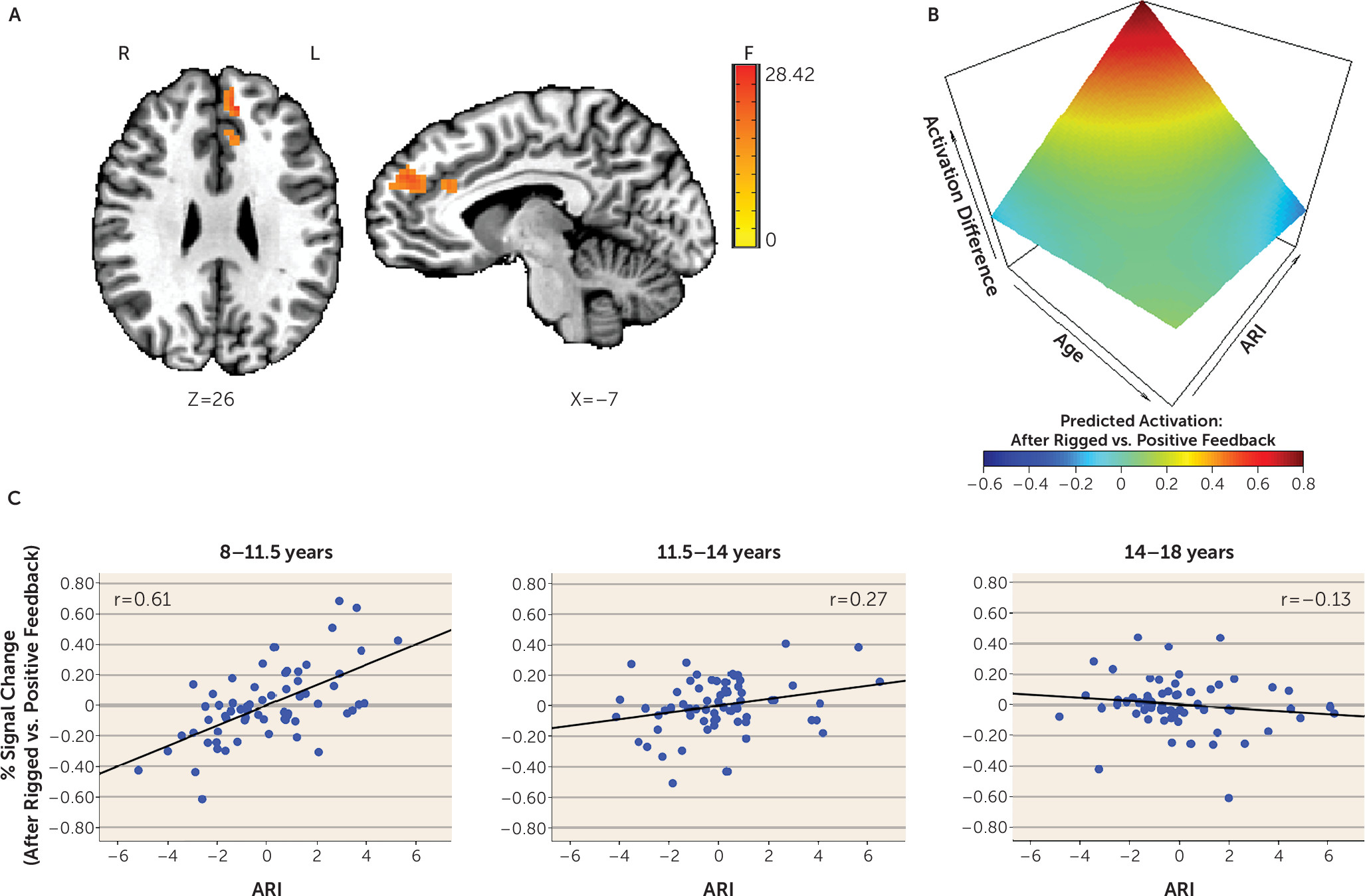
During the attentional event immediately following rigged feedback compared with positive feedback, activation in several posterior and frontal regions varied with levels of irritability and as a function of participant age. These regions include the cuneus, precuneus, superior parietal lobule, medial frontal gyrus, and anterior cingulate cortex (
Table 2,
Figure 2; see also Figure S3 in the
online supplement). Across these regions, higher irritability was more strongly related to increased activation in younger compared with older youths. Specifically, higher irritability was significantly related to increased activation in young children (ages 8–11.5) and younger adolescents (ages 11.5–14), with the strongest correlation in young children. However, irritability was not related to activation in older adolescents (ages 14–18). Findings in the medial prefrontal cortex and anterior cingulate cortex are summarized in
Figure 2.
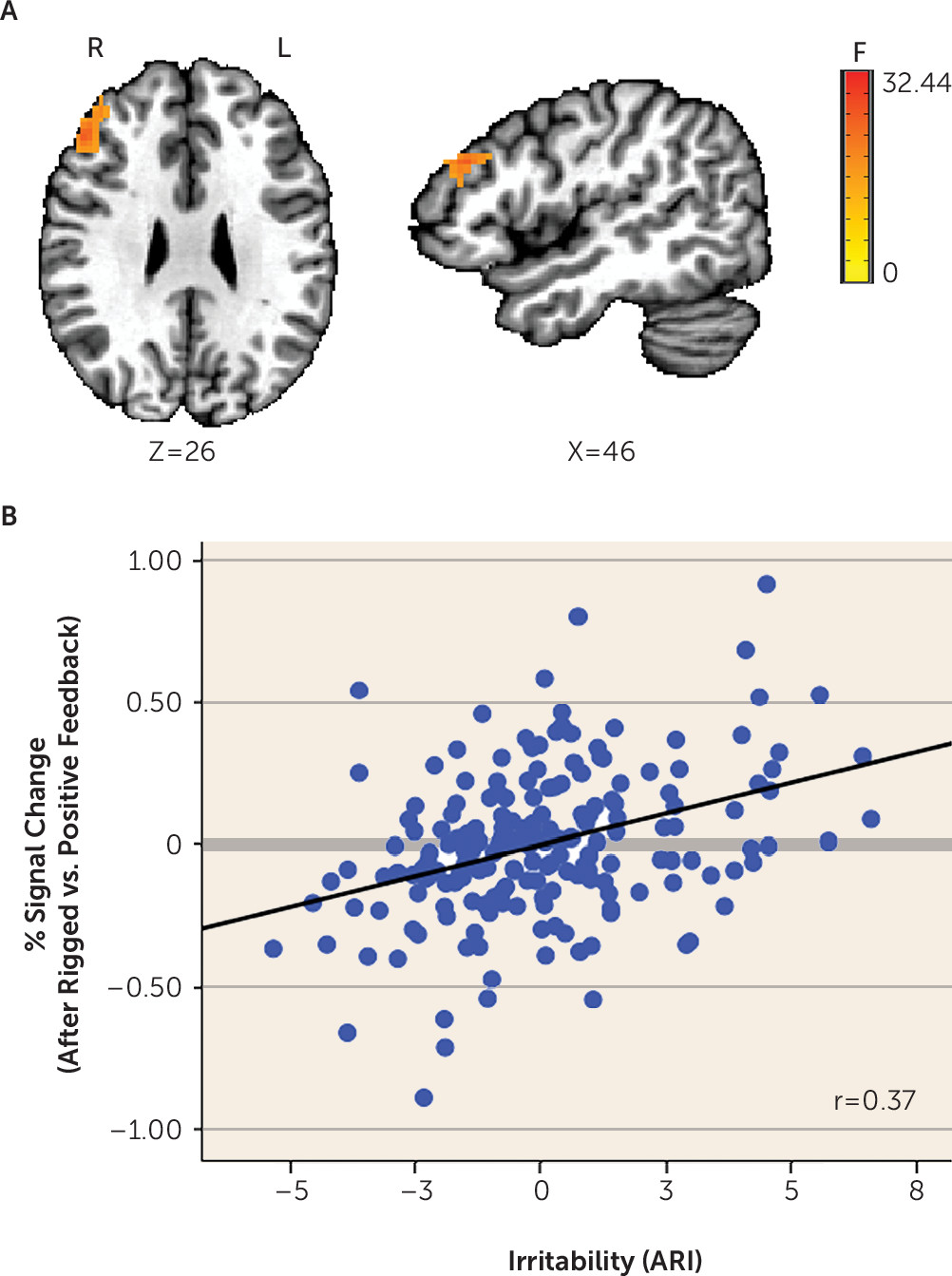
ARI-by-condition.
This two-way interaction yielded significant findings in the left and right cingulate gyrus, middle frontal gyrus, caudate, dorsolateral prefrontal cortex, cuneus, precuneus, and inferior frontal gyrus (
Table 2,
Figure 3; see also Figure S4 in the
online supplement). Most (91.6%) significant voxels in these regions did not overlap with the significant voxels for the three-way interaction described above. The few overlapping regions included the left and right cingulate gyrus, middle frontal gyrus, cuneus, and precuneus. Given that these regions were qualified by a three-way interaction of ARI by age by condition, we do not interpret them here. Regions showing a significant ARI-by-condition effect without a qualifying three-way interaction included the dorsolateral prefrontal cortex (
Figure 3), caudate (extending to the thalamus), and inferior frontal gyrus, among others (
Table 2; see also Figure S4 in the
online supplement). Across these regions, higher irritability was related to increased activation following rigged feedback compared with positive feedback.
Post Hoc Analyses: Depressive Symptoms, Frustration and Unhappiness Ratings, Medication, DSM-5 Diagnosis, and Gender
Given the longitudinal link between childhood irritability and depression later in life (
3), we evaluated the effect of depression by conducting whole-brain analyses with depressive symptoms (measured by the self-rated Children’s Depression Inventory [
35]) as the main dimension. There were no significant associations between depressive symptoms and neural activations (threshold voxel-wise p=0.001, cluster extent ≥703 mm
3). Depressive symptoms were evaluated this way and were not treated as a covariate in the main analyses (unlike anxiety and ADHD symptoms) because 28 participants had missing data.
We also conducted analyses to examine the effects of “state irritability” measured by self-ratings of frustration and unhappiness obtained at the end of each of the two frustration runs (see the Results section in the online supplement). The only significant finding was a positive association between unhappiness and activation in the right superior temporal gyrus during processing of rigged feedback compared with positive feedback. These analyses have limitations because of the problems associated with the state measures (see the Results section in the online supplement). Nonetheless, they suggest that the neural substrates underlying transient, subjective feelings of frustration and unhappiness may differ from the neural substrates mediating trait irritability.
We evaluated the confounding effect of medications by iteratively excluding participants by medication class (stimulants, antidepressants, and antipsychotics) (for further details, see Tables S3 and S4 in the online supplement). All significant findings remained. Additionally, analyses comparing medicated (N=25) with nonmedicated (N=100) participants on the day of the scan did not reveal significant between-group differences on the imaging findings.
Categorical analyses using diagnoses instead of symptom dimensions yielded null results in whole-brain activation but a few findings in functional connectivity (for further details, see the Results section in the online supplement).
We also examined the moderating effect of gender and found that most of the main imaging results were not moderated by gender. However, in the inferior parietal lobule, pre- and post-central gyri, and insula, irritability was related to increased activation in younger boys and decreased activation in older boys (see also the Results section in the online supplement).
Additional Region-of-Interest and Functional Connectivity Analyses
Region-of-interest analyses in the amygdala and striatum revealed positive associations between ARI and striatal activity during the N+1 trial but not during feedback (age moderated some associations) (see the Results section in the online supplement). We also analyzed functional connectivity with the inferior frontal gyrus and amygdala seeds (see the Results section, Tables S5 and S6, and Figures S5–S8 in the online supplement). Notably, we found that higher irritability was related to decreased functional connectivity between the left inferior frontal gyrus and periaqueductal gray (extending to the culmen) during the N+1 trial (see Figure S6 in the online supplement).
Discussion
This study investigated the neural correlates of attention orienting following frustration in a transdiagnostic sample of 195 youths with varying levels of irritability. We used a novel paradigm to model the frustration that irritable youths are prone to experience, particularly when reward is withheld and they are frustrated and asked to adjust their behaviors (e.g., to stop playing video games and start homework instead). We found that irritability was associated with neural activation during attention orienting following a frustrating event but not during processing of the frustrating feedback itself. Two specific findings emerged. First, higher irritability was related to increased activation in multiple frontal-striatal regions independently of age. Second, in other regions, associations between irritability and activation were moderated by age, such that associations were stronger in younger children compared with older children. These findings suggest that promising treatments for irritability may target frontal-striatal regions and that interventions could be prioritized for younger youths with high irritability.
Irritability was associated with dysfunction in the prefrontal cortex, anterior cingulate cortex (
20–
22,
36,
37), and striatum (
19,
36). This is consistent with previous research in youths and adults with irritability or related phenotypes (anger and trait aggression) (
19–
22,
36,
37). Specifically, while engaged in the attentional part of the task after being frustrated, highly irritable youths exhibited increased activation in multiple frontal regions (anterior cingulate cortex, dorsolateral prefrontal cortex, and inferior frontal gyrus) implicated in cognitive control, executive attention, and attention orienting (
38,
39). Highly irritable youths also showed increased activation in the striatum, which has a regulatory influence on the cortex and is involved in motor control and eye movements as well as in set shifting (
40).
Notably, irritability was not associated with performance deficits (e.g., poor accuracy and slower reaction time). Thus, the observed increased activation may reflect a compensatory mechanism (i.e., compared with healthy youths, irritable youths may require more robust recruitment of these regions following frustration to regulate their negative affect, focus on the task at hand, and meet task demands). Importantly, our finding is best explained by irritability and not better attributed to co-occurring anxiety, ADHD, or depressive symptoms. Furthermore, we found no associations between irritability and activation during non-frustration runs. This observation suggests that trait-specific neural correlates manifest in irritable youths when they are frustrated. Such context specificity is consistent with our event-related analysis contrasting activity during performance of the attention-orienting task following frustrating feedback compared with non-frustrating feedback. Because of the insufficient number of incorrect trials, we were unable to examine the neural responses to errors (in which reward is omitted and not expected). An interesting and important question is whether irritability has similar associations with responses to errors, responses to “pure” frustrative nonreward (i.e., in which reward is expected and omitted because of changed contingencies), and responses to frustrative nonreward due to deception (rigged feedback).
Despite comparable performance on a simple attention task, irritable youths exhibited heightened frontal activation following frustration, suggesting a requirement for greater prefrontal cortex engagement to adjust to frustration and achieve performance comparable to that of less irritable youths. Many everyday tasks (e.g., schoolwork, homework, or transitions between activities) are much more cognitively demanding than laboratory tasks, such as the one used here. Therefore, inefficiency in systems that facilitate post-frustration adjustment could lead irritable youths to struggle in daily life. Moreover, whereas many irritable youths in our sample were medicated, the impact of medications on their brain function was not evident in our analysis. This suggests that current medications may fail to normalize the particular neural dysfunction reported here. There is clearly a need for new treatments, including nonpharmacological approaches (e.g., real-time fMRI neurofeedback or transcranial magnetic stimulation targeting frontal-striatal regions). Additionally, given that neural dysfunction associated with irritability was found during attentional processes following frustration, intervention efforts might emphasize strategies that help irritable children allocate attention effectively.
Age moderated the association between irritability and brain activation during attention orienting following frustration in several frontal and posterior regions (anterior cingulate cortex, medial frontal gyrus, cuneus, and precuneus). Specifically, higher irritability was related to increased activation in children and younger adolescents but not older adolescents; the association was particularly strong in young children. This finding is inconsistent with the only previous study that examined the interacting effect of age and irritability, which found that as age increased, higher irritability was associated with more frontal-striatal-thalamic activation (
17). This discrepancy could be explained by differences in sample size (N=30 compared with N=195), sample characteristics such as age (4–12 years compared with 8–18 years), and the nature of the sample (nonclinical compared with clinical) as well as fMRI paradigm (emotionally or neutrally valenced video clips compared with a frustrating cued-attention task). Young and highly irritable children may be most susceptible to affectively charged stimuli and may have the greatest difficulty disengaging attention from negatively arousing stimuli. Although these youths did not show performance deficits, their ability to perform the task despite immature neural circuitry may have required prolonged and inefficient computational processes that generated increased regional neural activity (
41). Indeed, the increased anterior cingulate cortex activation seen in young children, compared with older youths and adults, may reflect neural inefficiency in cognitive control (
41).
Contrary to some previous studies, we did not find an association between irritability and amygdala activation in response to a frustrating event (
19,
37). This could be due to differences in frustration paradigms (i.e., a block design [
37] compared with an event-related design, or no jitter [
19] compared with jitter between attentional and feedback portions of the task [the latter allows for separation of attention orienting and feedback processing]). Alternatively, the unreliability of amygdala activation may hamper replication (
42). It is noteworthy that our null amygdala finding is consistent with a recent study that also adopted a dimensional approach to examine irritability and brain function during frustration (
20).
There are several limitations to our study. First, our findings may apply only to the disorders sampled (disruptive mood dysregulation disorder, anxiety disorder, and ADHD) and not to other disorders (e.g., depression and bipolar disorder) in which irritability is also common. Second, given the high correlation between chronological age and puberty status, we used age as a proxy for development. Future work is needed to directly examine the effect of puberty. Third, although our cross-sectional design is a helpful starting point to understand brain function over development, only longitudinal studies can elucidate individual differences in developmental trajectories. Fourth, as in most studies of youths with severe impairments, medication may confound the results; however, our post hoc analyses do not support confounding by medication. Finally, this study used raw, reported scores of irritability symptoms. Other phenotyping approaches (e.g., latent variable modeling) may provide additional perspectives.
Conclusions
In a sample characterized by a mix of mood, anxiety, and ADHD symptoms in the clinically impairing range, we found unique associations of irritability with neural systems mediating attention orienting, top-down regulation of emotions, and motor execution following frustrative nonreward. These associations were not attributed to co-occurring ADHD, anxiety, or depressive symptoms. Although most associations were independent of age, dysfunction in the anterior cingulate cortex and some posterior regions was more pronounced in young children with irritability. The neural dysfunction did not seem to be altered by medications, highlighting a need for new treatments, including nonpharmacological approaches, that target the common and impairing symptom of irritability. Because associations between irritability and brain function were found during attentional processes following frustration, intervention efforts could target strategies that help children with irritability regulate the negative affect and arousal elicited by frustrating events and flexibly shift their attention to focus on the task at hand.
Acknowledgments
The authors thank the children and families who participated in this study as well as the staff of the Emotion and Development Branch and the Scientific and Statistical Computing Core at NIMH. The authors also thank Anderson Winkler, M.D., D.Phil. (from the NIMH Emotion and Development Branch) for assistance on permutation analysis of linear models and threshold-free cluster enhancement analysis, Dan Barlow for programming support, and Gang Chen, Ph.D., for imaging statistics guidance.