Obsessive-compulsive disorder (OCD) affects 1%−3% of children and adolescents and 2%−3% of adults, and is characterized by recurrent and intrusive obsessive thoughts that patients attempt to neutralize with behavioral and/or mental compulsions (
1). Psychological treatment for OCD consists primarily of cognitive-behavioral therapy (CBT) incorporating in vivo exposure and response prevention (
1). During exposure and response prevention, patients interact with symptom-provoking stimuli while resisting compulsions, thereby learning that compulsive rituals are not necessary to prevent feared outcomes (
2). Meta-analyses show large effect sizes for symptom severity reductions following CBT, even when compared with active control psychotherapies (
1,
3). However, approximately 30%−50% of patients with OCD do not respond adequately to treatment, and reliable predictors of response to treatment have yet to be established (
3).
The neural mechanisms underlying OCD remain poorly understood, but cingulo-opercular and orbito-striato-thalamic networks are commonly implicated in patients across the lifespan (
4). OCD patients show impaired performance as well as hypoactivation within cingulo-opercular regions during cognitive control, identifying possible mechanisms of impaired control over obsessions and compulsions (
4,
5). The orbito-striato-thalamic network appears hyperconnected at rest and hyperactive during symptom provocation and habit-driven responding in the disorder, whereas orbito-striatal regions are hypoactive during reward processing and decision making, suggesting an imbalance of habit and goal-directed functions within these regions in OCD (
6–
8). Other work has linked cingulo-opercular and orbito-striatal activation during cognitive control and reward processing with treatment response to CBT (
9,
10). Greater cingulo-opercular functioning during cognitive control may indicate a greater ability to engage this network during self-regulation to implement response-prevention strategies (
10). Orbito-striatal and connected limbic regions are key for maintaining motivation as well as for learning new associations for environmental stimuli and behavioral actions, as required during exposure and response prevention (
11,
12).
Previous studies have examined whether individual differences in pretreatment brain activation or structure are associated with treatment response to CBT in OCD, implicating cingulo-opercular, orbito-striatal, and amygdalar regions (
13–
15). One study (
10) reported that adult patients with more pretreatment activation during cognitive control within cingulo-opercular, dorsolateral prefrontal, posterior cingulate, striatal, and temporal regions had a better response to CBT. However, the existing literature has limitations. First, published neuroimaging studies have not included control psychotherapy groups and have not been able to separate findings associated with symptom change due to CBT from nonspecific symptom reduction (
10,
16). Findings of treatment-specific associations are critical in developing a mechanistic understanding of CBT, as well as in developing individually tailored treatment algorithms (
14,
15). Second, neuroimaging studies of treatment response in patients with OCD have focused primarily on adults. Since early intervention may well have advantages in disorders like OCD that are often early in onset, understanding whether treatment-specific predictors of recovery generalize beyond mature adulthood is important (
1,
9).
Therefore, in this study, we explored the associations between task activation and treatment response to CBT compared with a control psychotherapy—stress management therapy (SMT)—in adolescent and adult patients with OCD. Effective engagement with CBT likely places greater demands than control therapies on self-regulatory processes, which allow patients to control emotions, cognitions, and behaviors in the face of symptom triggers (
10). It also requires the capacity for maintaining the motivation to work toward long-term goals (e.g., symptom recovery) in the face of challenging exposures (
9,
12). Consequently, we anticipated that more pretreatment activation within cingulo-opercular regions during cognitive control and orbito-striatal regions during reward processing would be associated with larger symptom reductions in the CBT group, and that these associations would be treatment specific relative to SMT. Secondary analyses tested whether associations remained in both adolescent and adult subgroups.
Discussion
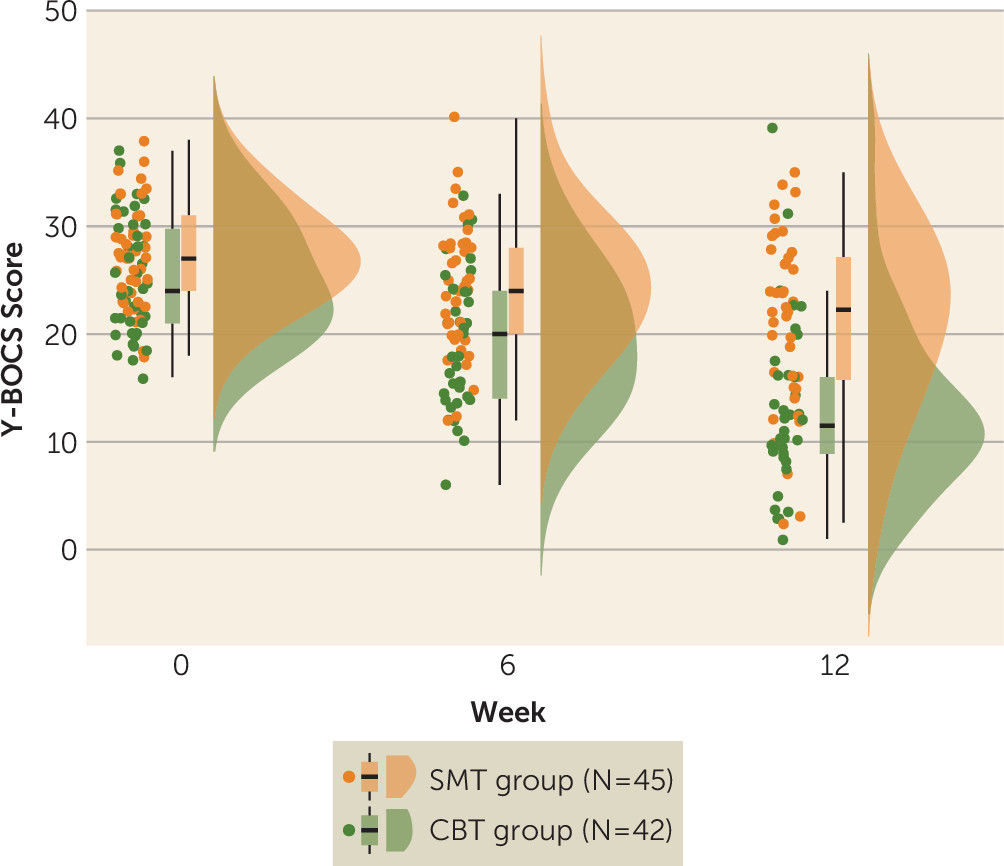
In this study, we sought to examine whether pretreatment brain activation during cognitive control and reward processing is associated with treatment response to CBT in patients with OCD, and if so, whether the associations are treatment specific relative to an active control therapy (SMT) and whether they vary with age. Patients in both treatment groups showed significant reductions in OCD symptoms after treatment, but symptom reduction following CBT was steeper than symptom reduction following SMT. In the CBT group, a pattern of greater pretreatment activation during cognitive control and reward processing was associated with a better treatment response, while relatively less activation in these regions prior to treatment was associated with better outcomes after treatment in the SMT group. Follow-up analyses in adolescent and adult subgroups indicated that findings were conserved across age.
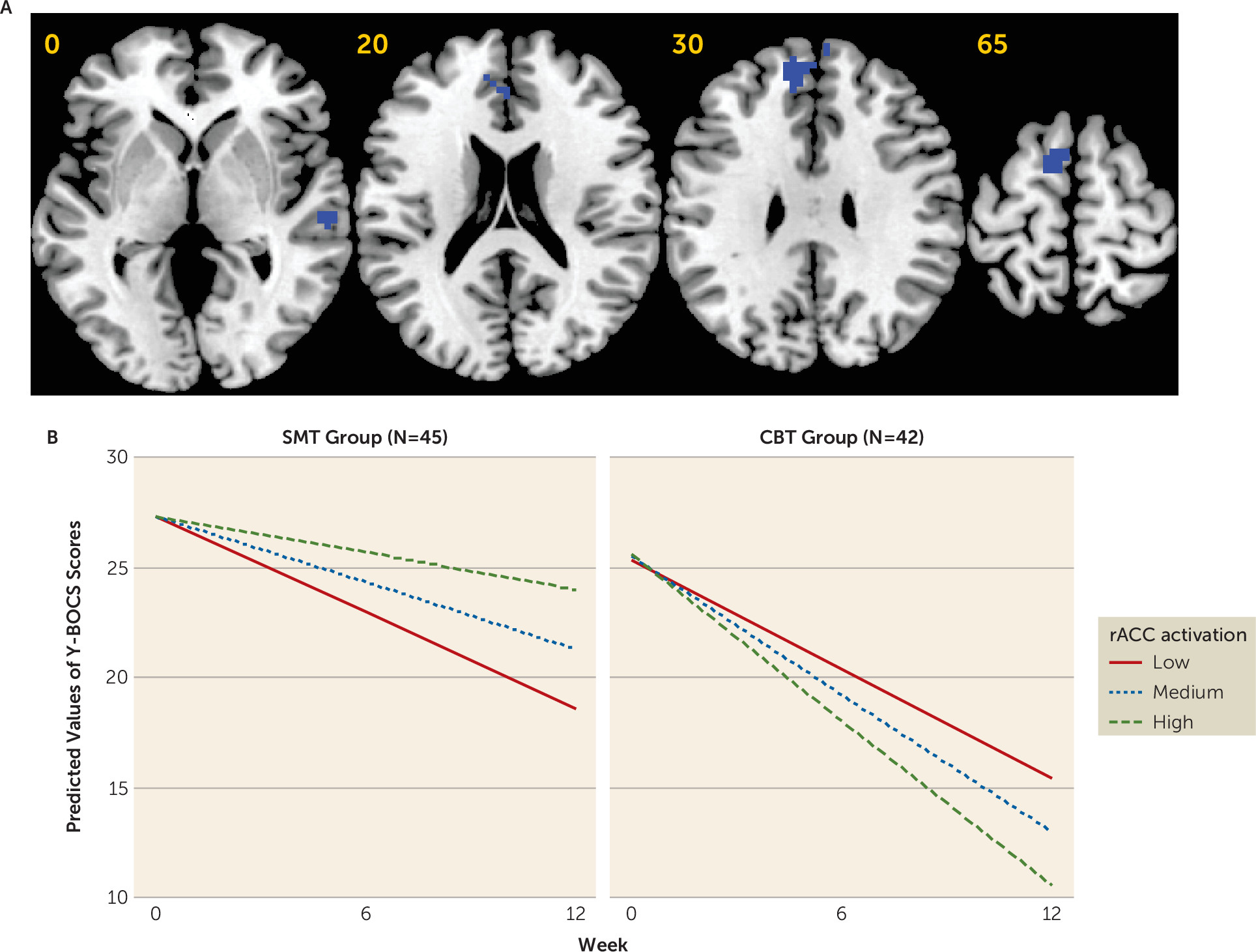
Greater symptom reduction after CBT treatment was associated with more baseline brain activation within the right temporal lobe and, at a relaxed cluster-forming threshold, within a hypothesized cingulo-opercular region, the rACC, that has been shown in meta-analyses to be reduced in gray matter volume and hypoactive during cognitive control in patients with OCD (
4,
5). These findings replicate a recent study of treatment response to CBT in adults with OCD (
10) and extend previous work by showing treatment specificity relative to SMT. Interestingly, in the CBT subgroup analysis, greater activation within anterior insular, dorsolateral prefrontal, and posterior cingulate regions was also associated with treatment response, providing further independent replication of this previous study, although these findings did not survive correction for multiple comparisons in the interaction analysis, leaving their treatment specificity unclear (see the
online supplement). Meta-analytic studies have shown that reduced structure and function of the rACC is commonly implicated across multiple psychiatric disorders (
5,
25,
26), and greater volume or activation within the rACC is arguably the most common predictor of a better treatment response to CBT (
27,
28). The rACC is proposed to play important roles in flexible top-down control of emotions and self-regulation in response to potential symptom triggers, cognitive-affective functions which are critically involved in CBT (
29). These findings, together with a converging research literature, suggest that patients with relatively preserved functioning within brain regions supporting cognitive control may be better candidates for CBT (
10).
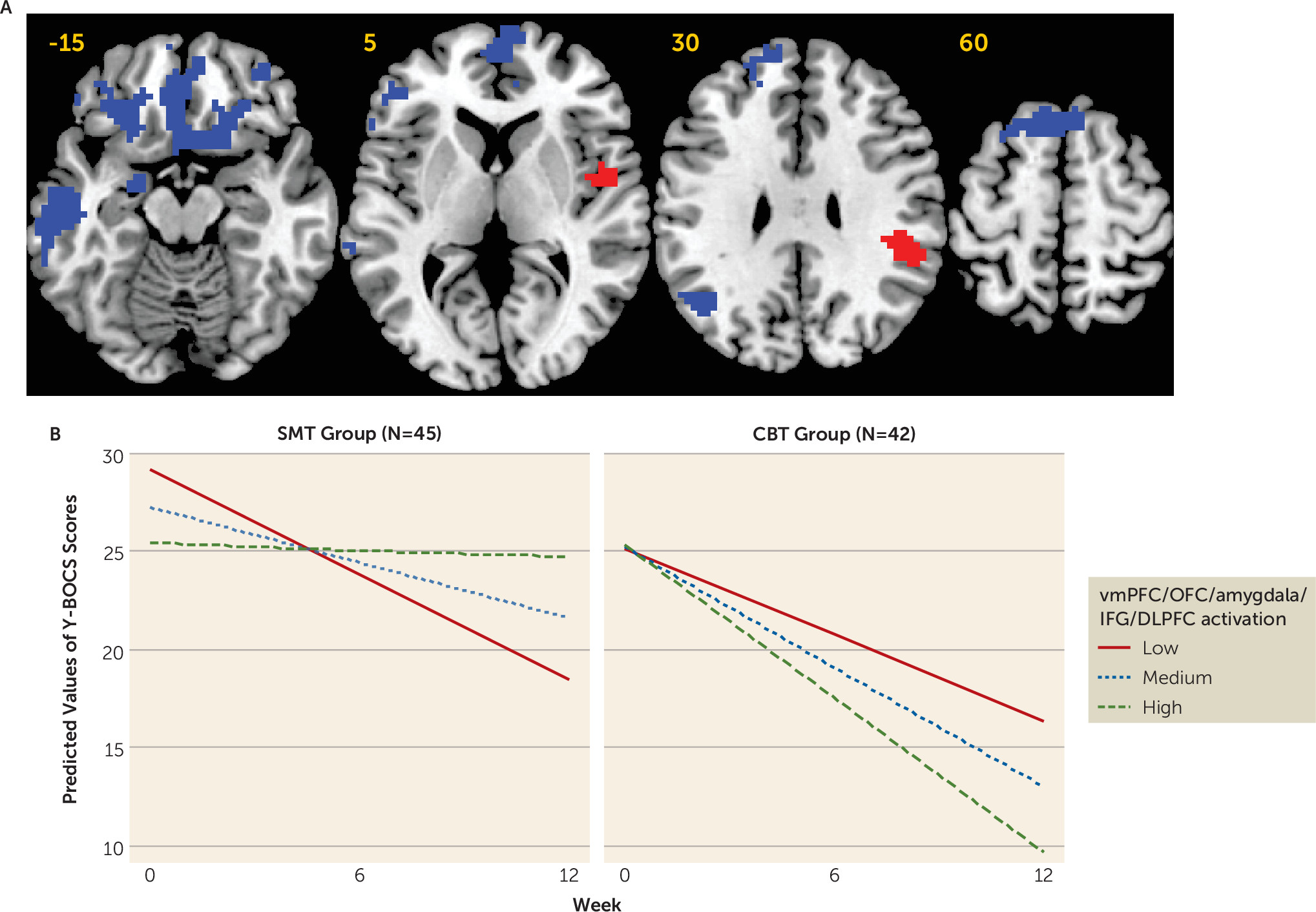
During reward processing before treatment initiation, greater activation in the left and right ventromedial prefrontal cortex/orbitofrontal cortex, lateral prefrontal, and amygdalar regions predicted better response to CBT. Recent work has suggested parallels between reward processing and fear extinction, as experiencing the presentation of a conditioned stimulus without the aversive unconditioned stimulus is a better than expected and therefore quasi-rewarding outcome, and studies in humans and animals have demonstrated that functioning within mesolimbic dopaminergic circuitry during extinction learning mirrors that seen during reward-related tasks (
11). Fear extinction learning is likely a key mechanism of exposure and response prevention for OCD, and therefore patients with relatively robust orbito-striato-limbic brain activation may be better able to learn updated and less negative associations for their symptom triggers while undergoing exposure and response prevention (
2,
11). More broadly, robust functioning within reward-processing regions may protect against impairments in motivation (e.g., anhedonia) and positive affect, which have been shown to have a negative impact on response to treatments, including CBT, across multiple disorders (
12,
30). Therefore, relatively greater pretreatment activation in patients who respond strongly to CBT may also indicate a greater capacity for emotional resilience and the motivation to engage in challenging aspects of CBT therapy (
12).
Interestingly, interaction analyses showed that while more brain activation within cognitive-control and reward-processing regions was associated with a better treatment response to CBT, a better treatment response to SMT was associated with less activation in an overlapping set of brain regions. While these findings in the SMT group were unexpected, they are consistent with studies comparing CBT with pharmacotherapy treatments, which have reported increased ventromedial prefrontal cortex/orbitofrontal cortex gray matter and resting-state metabolism to be associated with a better response to CBT, and the opposite for pharmacotherapy (
13,
15). One possibility is that CBT is most effective in patients who already possess the degree of cognitive control and reward responsiveness required for engaging with and learning from exposure and response prevention (
10,
11). SMT, on the other hand, may improve OCD symptoms indirectly by teaching patients how to relax and employ problem-solving techniques to reduce negative emotions in the face of common (i.e., non-OCD-specific) life stressors; thus, SMT may bring about therapeutic change via improved self-regulation and feelings of self-efficacy (
20). Consequently, SMT may be better able to meet the needs of patients who have the most room for improvement in these domains. However, given that these findings were unanticipated, further work is needed to properly delineate the mechanisms driving these findings in the SMT group.
Findings of treatment-specific associations suggest that rather than being a general correlate of symptom reduction, greater activation during cognitive control and reward processing is likely linked in a more specific way to CBT response in patients with OCD. We have demonstrated in recent meta-analytic work that patients with OCD show impaired performance and reduced activation within the rACC during cognitive control (
4,
31). Moreover, hypoactivation during reward processing has been reported in patients with OCD in prefrontal and orbito-striato-limbic regions similar to those associated with treatment response in the present study (
8,
32). Findings indicate that treatment response to CBT depends on circuitry known to be dysfunctional in OCD, suggesting that engagement with CBT might be augmented by pharmacological or nonpharmacological treatments that target these underlying networks (
25), and that it may be possible to identify good candidates for CBT on the basis of relatively preserved functioning in these brain regions during cognitive control and reward processing (
14).
In the present study, analyses in age-defined subgroups indicated that the same pattern of treatment-specific associations was present in both adolescent and adult patients. The findings provide initial evidence for a preservation of neural predictors of CBT response across the lifespan. However, all patients in our study had early-onset forms of the disorder. OCD has a bimodal onset distribution, with peaks at around age 10 and age 20, and early- and late-onset forms of the disorder have been linked to distinct clinical, neuropsychological, and neurobiological correlates, as well as distinct treatment outcome trajectories (
33). Future research should examine whether there are differences in brain activation during cognitive control and reward processing between early- and late-onset forms of the disorder, and whether there are distinct neural predictors of treatment response in these patient subgroups. No differences in activation were found between adolescent and adult patients during cognitive control and reward processing, unlike in some studies in healthy subjects (
34). However, interpreting this as evidence of altered development in the disorder would be problematic, as findings in healthy subjects have been mixed and no normative data on the development of activation during the incentive flanker task have been published (
4,
34).
Limitations of this study include the fact that, although outcome assessments were made blinded to treatment group, it was not possible to maintain blinded status for patients. This issue is common to previous clinical trials comparing CBT with SMT (
2,
19). In addition, we excluded patients with common comorbidities, and the findings may not generalize to patients with these comorbid conditions. Furthermore, negative findings may be due to relatively lower power for the error processing contrast because of the smaller number of trials. Moreover, the sample size of the study was moderate, and while the present findings provide initial evidence for the potential of using task-based fMRI in distinguishing good and poor responders to CBT and SMT, before translation to the clinic these findings must be shown to be robust, replicable, and generalizable to other patient subgroups, as well as to other treatment and imaging sites. Although similar findings were found for adolescents and adults, the inclusion of two age groups added heterogeneity to the sample. While the study findings reveal treatment-specific outcome predictors in patients with OCD undergoing CBT or SMT, they do not speak to whether these predictors are conserved across different disorders that are treated with similar therapies and in which similar cingulo-opercular and orbito-striatal regions have been implicated (
25,
30).