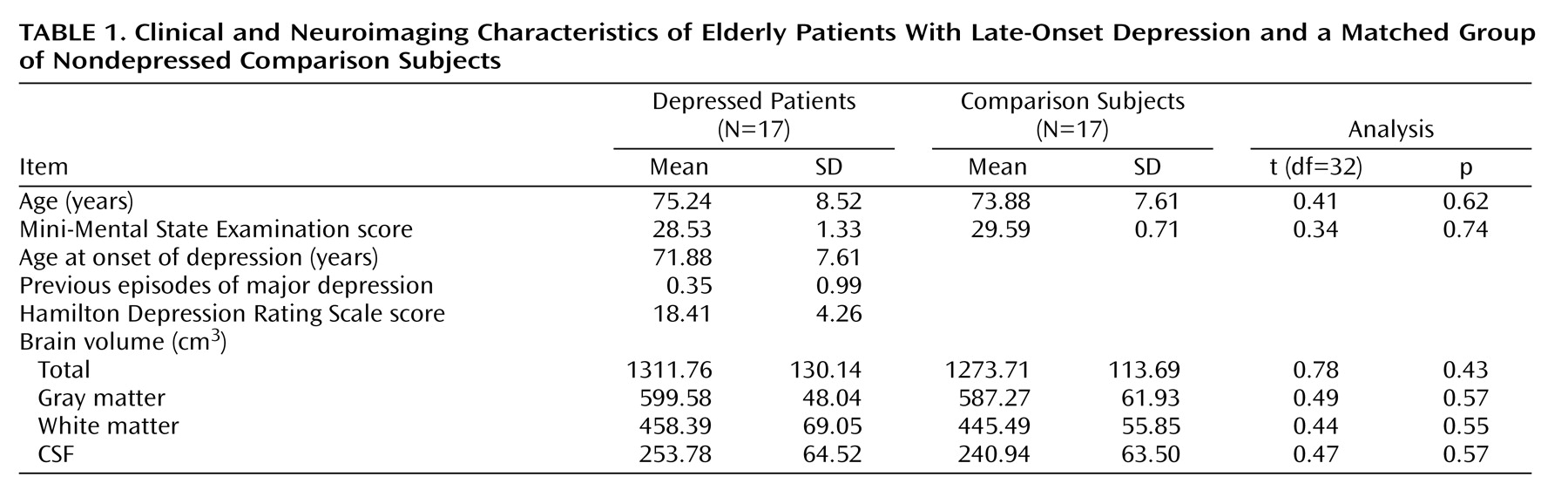
In a subset of elderly patients with major depression, the first episode occurs late in life
(16). This subgroup, frequently referred to as having late-onset depression, exhibits certain unique clinical, biological, and neuroimaging characteristics
(17–
22). More specifically, late-onset depression patients are less likely to have psychiatric comorbidity
(23) or a family history of depression
(20,
24) when compared with early-onset depression groups. On the other hand, late-onset depression patients are more likely to have associated medical comorbidity
(23,
25–28), greater cognitive deficits
(29), and increased risk of developing dementia
(22). They also more frequently experience a loss of interest and apathy
(19,
21).
Previous structural neuroimaging studies that focused on late-onset depression have employed traditional volumetric analyses where regional boundaries must be identified. While these studies have provided some evidence for regional specificity of volume loss in late-onset depression, the question of spatial localization of gray matter deficits over the entire cortical surface in late-onset depression has not been addressed in the literature. Such information could provide more comprehensive insights into the neuroanatomical contributions to late-onset depression.
The goal of the present study was to use cortical pattern matching methods in order to perform detailed spatial analyses of gray matter abnormalities on lateral and interhemispheric brain surfaces in subjects with late-onset depression relative to group-matched comparison subjects. These brain mapping methods allow data from each individual to be analyzed with a series of manual and automated procedures that carefully match cortical anatomy across subjects and measure local proportions of gray matter at each of 65,536 anatomically matched points on the cortical surface of each cerebral hemisphere. An advantage of this technique is that subtle as well as spatially diffuse differences in regional gray matter can be identified over the entire cortical surface by closely matching structurally homologous brain regions across subjects. This is made possible through definition of cortical sulcal landmarks on the brain surface of each individual.
We set out to examine whether regionally specific patterns of gray matter abnormalities are present in late-onset depression patients relative to nondepressed subjects of comparable age and gender, after carefully matching gyral anatomy across subjects. On the basis of the existing literature suggesting that late-onset depression is more likely to be associated with cortical atrophy, and considering our previous findings, we expected that late-onset depression would exhibit gray matter deficit patterns in frontal, temporal, and parietal cortices.
Discussion
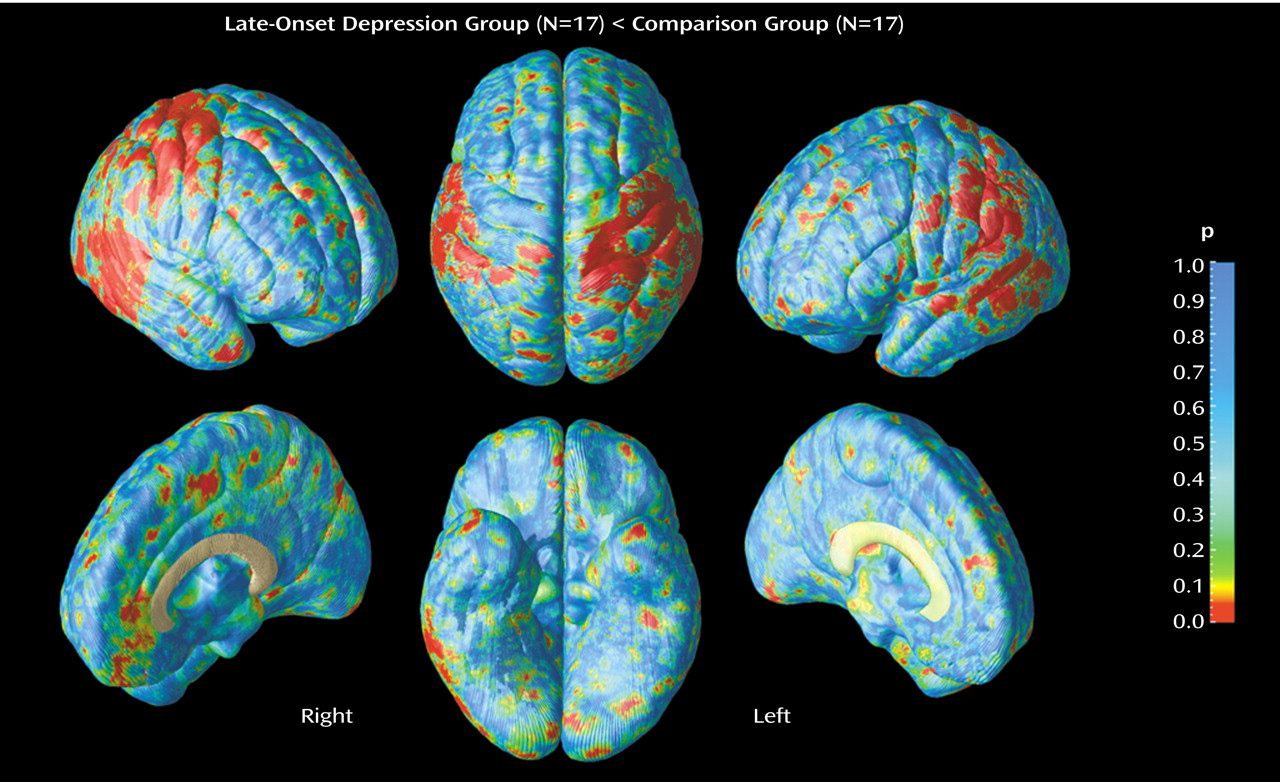
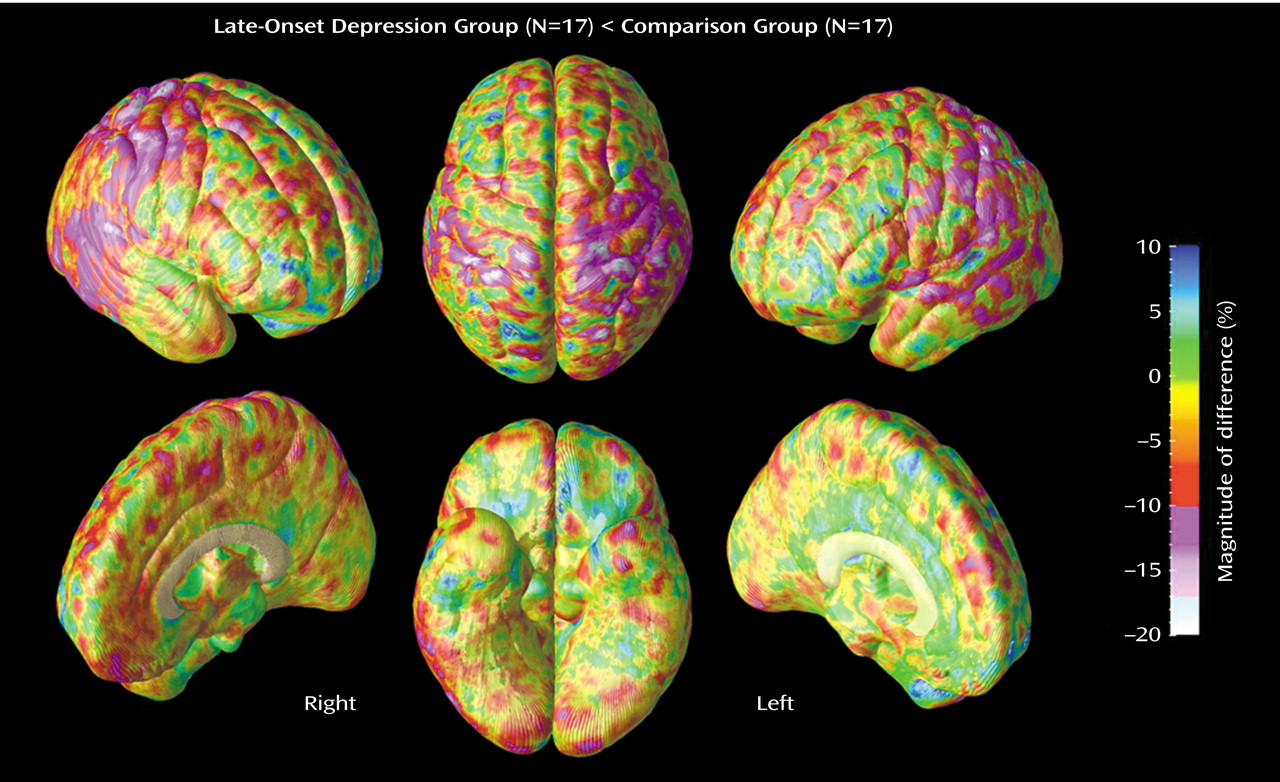
Two main positive findings emerged from our study: 1) significant gray matter deficits, confirmed with permutation testing, in the right lateral temporal cortex, mainly the posterior portions of the superior and inferior temporal gyrus, and 2) prominent decreases of gray matter in the right parietal cortex, where deficits were most pronounced in sensorimotor regions, again confirmed with permutation tests. In the same regions, similar patterns of gray matter deficits also appeared in the left hemisphere that approached significance with permutation testing.
Several structural MRI studies have demonstrated hippocampal or temporal lobe abnormalities in late-life depression
(11–
14), whereas others did not observe significant differences
(1,
3,
53). Of all these studies, two focused on patients with first onset of depression after the age of 60
(3,
13), with one showing significantly more left medial temporal atrophy than early-onset patients
(13), and the other showing differences in total temporal lobe volume that approached significance between patients with late-onset minor or major depression and comparison subjects
(3). Manual delineation of the entire temporal lobe is difficult because the orientation and anatomic boundaries can be unclear. This may at least in part explain why in our previous study decreases in total temporal volume only approached statistical significance and why most region of interest studies examined specific temporal subregions such as the hippocampus, which can be more easily identified. A study by Steffens et al.
(14) suggested that hippocampal volume atrophy may be greater in late-onset depression compared with early-onset depression. It is interesting that the anatomical regions within the temporal lobe where we saw the most pronounced gray matter deficits were the posterior aspects of the superior and inferior temporal gyrus, without any differences in ventral/medial areas. Although our study supports the hypothesis that temporal lobe atrophy is associated with late-onset depression, further studies are clearly warranted to examine which specific temporal subregions are most relevant in the context of late-onset depression.
In addition to our findings of gray matter decrease in lateral temporal regions, the present study is also the first to report gray matter deficits in parietal cortices, mainly sensorimotor regions, in late-onset depression. Lesions in the right temporal or parietal cortices have been shown to impair emotional experience and arousal
(54–
56) and to impair imagery for emotion
(57–
60). In addition, patients with tumors affecting temporoparietal cortices have reported pronounced negative mood states after surgery
(61). There is evidence that both temporal and parietal cortices contribute to integrating viscerosensory information with affective signals
(62,
63). Functional imaging studies in major depression have documented changes in regional cerebral flow or metabolism in the temporal cortex
(64–
66) as well as in the parietal cortex
(67,
68). In geriatric depression, reduced superior temporal and anterior parietal activations have been reported
(69).
Using cortical pattern matching methods, we also detected gray matter abnormalities in temporoparietal cortices in our previous report
(9) that focused on elderly depressed patients with an early age at onset. However, widespread increases of gray matter instead of decreases were found in these regions. Furthermore, the distribution of gray matter changes in temporal lobe structures differed somehow from the pattern identified in late-onset depression, since gray matter increases were more pronounced in the left hemisphere and observed throughout the lateral surface, including the temporal poles, rather than being more right-lateralized and localized in more dorsal lateral aspects, as observed in late-onset depression.
Whereas gray matter increases may reflect abnormal neurodevelopmental processes, perhaps because of an excess of gray matter early in development that decreases over time yet remains above the normal threshold, the reduction of gray matter in late-onset depression is more likely to be associated with neurodegenerative events. It remains to be clarified whether the region-specific gray matter deficits observed here in late-onset depression are necessary for initiation of the illness or instead represent an epiphenomenon that perhaps appears with progressive accumulation of the number of stressors in later life (i.e., neuronal loss due to the aging process, concomitant medical illness, and grief).
Contrary to our predictions regarding frontal gray matter deficits in late-onset depression, no significant group differences were found throughout this anatomical region. Several previous MRI volumetric studies that implicated structural abnormalities of the frontal lobe, particularly the orbitofrontal cortex, in geriatric depression
(1,
5,
8,
70) mainly included patients with long histories of depression, and it is not clear what proportion of their samples had late-onset depression. Of the three previous MRI studies focused on late-onset depression (defined as illness onset after age 60), all examined frontal lobe atrophy using traditional volumetric methods, although results remain mixed
(3,
7,
31). Indeed, in an earlier report by our group
(3), late-onset depression was associated with greater bilateral frontal atrophy relative to that of nondepressed comparison subjects. However, in a subsequent study
(31) we reported that lobar structural abnormalities in the frontal regions did not change with an increasing age at onset of illness. Instead, Almeida et al.
(7) found that patients with late-onset depression had significantly more right frontal atrophy than their early-onset counterparts of similar age, without observing significant differences between late-onset depression and nondepressed comparison subjects. However, our results are not fully comparable with previous region of interest studies in late-onset depression, given that these studies examined frontal lobe volumes as a whole and did not specifically address gray matter differences. It is therefore possible that subcortical abnormalities, such as white matter lesions, may have contributed to the evidence of atrophy in total frontal volume in previous reports. For example, one study showed that subcortical lesions may decrease orbitofrontal cortex volume in geriatric depression
(70). Although white matter lesions were not specifically addressed in the present study, global white matter volumes did not differ between groups (
Table 1). Future studies are needed to understand how subcortical lesions may be related to cortical gray matter changes. Indeed, based on the results of an earlier report
(71), comorbid medical disorders may have only a modest impact on brain volume reductions. The differences in the results between our earlier region of interest study in late-onset depression
(3) and the present report may additionally arise from differences in the demographic or clinical characteristics of the populations of depressed patients examined. While our earlier study included inpatients, the patients in the study reported here were exclusively outpatients and had an average of less than one prior major depressive episode.
Regarding frontal cortices, it is noteworthy that the results in late-onset depression differ from the pattern identified among elderly patients with early-onset depression in previous research that used computational cortical pattern matching
(9). More specifically, the latter study showed a prominent regional size reduction in the orbitofrontal cortex, together with significant increases in cortical gray matter adjacent to focal decreases of gray matter in the same region. In late-onset depression, we expected to find gray matter loss rather than gray matter increase in frontal regions because this subtype of depression is thought to be associated with greater cortical atrophy than early-onset groups
(22).
Although the division between “early” and “late” onset is still considered somewhat arbitrary and not based on reliable clinical and biological data, it remains possible that the patients in the study reported here may not show discernible frontal gray matter deficits because such abnormalities could require more prolonged or intense exposure to potentially neurotoxic events. Whether and to what extent disease severity or longer total history of depression might contribute to frontal abnormalities is yet to be unraveled. On the other hand, there is evidence that the effects of hypercortisolemia in major depression exhibit regional specificity in cortical regions, with intensity of these effects differing according to anatomical site
(72). Temporal and parietal cortices of late-onset depression may therefore be, for some reason, more sensitive to the potentially toxic effects of such insults.
There are now a number of studies supporting the view that late-onset depression patients are more likely to have associated cognitive impairment and are more likely to progress to dementia
(22). None of our patients had a history of mental status examination results suggesting dementia, although it is possible that some of them will develop progressive cognitive impairment over time
(73). Nevertheless, it is noteworthy that the gray matter changes observed here do not show the same patterns as the changes seen in patients with Alzheimer’s disease. Indeed, previously published longitudinal Alzheimer’s disease studies using cortical pattern matching or voxel-based morphometry demonstrated that gray matter loss appears in ventral/medial regions of the temporal cortex in the earliest stage of the illness
(41,
74), whereas here no group differences were detected in these regions. Furthermore, sensorimotor regions are relatively spared in Alzheimer’s disease
(41,
51), whereas these are the regions within parietal cortices where the gray matter deficit was most pronounced in late-onset depression. This may suggest that the gray matter abnormalities reported here might be indeed disease specific.
Limitations of this study should be noted. The study groups were relatively small, a consideration that suggests caution when interpreting results. That is, it is possible that we did not have sufficient power to detect subtle differences in gray matter that may exist in other cortical regions in late-onset depression. Notwithstanding, since this study is, to our knowledge, the first to identify specific gray matter deficits in temporal and parietal cortices in late-onset depression, replication with a large homogenous group of subjects may serve to confirm the findings reported here. In addition, approximately two-thirds of the subjects were women. We corrected for potential interindividual differences in brain size that might be associated with gender by performing our analyses in International Consortium for Brain Mapping-305 space. However, future studies may be needed to investigate the specific contribution of gender to gray matter abnormalities in late-onset depression. Information on handedness was not available. Future research may further investigate laterality effects, given that larger gray matter differences were observed in the right hemisphere. Our findings in the present context, however, may reflect that power is lacking and not necessarily that laterality effects are present.
Measurements of gray matter obtained after cortical pattern matching algorithms are constrained by size of the sphere used to sample local gray matter proportions. In this study, we chose a 15-mm kernel size to increase signal to noise for local measurements of gray matter
(41,
42,
49). Previous studies demonstrated that tissue proportion measurements using this kernel size are comparable to those found with manual delineation procedures. Indeed, Sowell et al.
(75) documented that measures of gray matter concentration and gray matter volume are similar when the manually delineated region of interest is small and at the same cortical surface location.
In conclusion, the sensitive cortical pattern methods employed in this study were able to detect prominent local gray matter deficits in the right lateral temporal cortex and parietal cortex, most pronounced in sensorimotor areas, in subjects with late-onset depression relative to nondepressed comparison subjects. These novel findings imply specific neurodegenerative processes in late-onset depression that differ from the pattern commonly seen in dementia. Only longitudinal follow-up evaluations, however, will be able to make this distinction, with important implications from both therapeutic and research standpoints.