Lower Ventral Striatal Activation During Reward Anticipation in Adolescent Smokers
Abstract
Objective:
Method:
Results:
Conclusions:
Method
Characteristics of the Multisite Study
Participants
Personality and Neuropsychological Measures
Delay Discounting
Modified Monetary Incentive Delay Task
Analysis of Behavioral Data
fMRI Data Acquisition
fMRI Preprocessing and Analysis
Results
Personality and Demographic Characteristics
| Characteristic | Comparison Group (N=43) | Smokers (N=43) | |||
|---|---|---|---|---|---|
| N | N | ||||
| Sex | |||||
| Male | 24 | 24 | |||
| Female | 19 | 19 | |||
| European School Survey Project on Alcohol and Drugs (18) | |||||
| Number of lifetime occasions of smoking | |||||
| None (score=0) | 43 | 0 | |||
| 1 or 2 (score=1) | 0 | 2 | |||
| 3–5 (score=2) | 0 | 5 | |||
| 6–9 (score=3) | 0 | 7 | |||
| 10–19 (score=4) | 0 | 4 | |||
| 20–39 (score=5) | 0 | 4 | |||
| 40 or more (score=6) | 0 | 21 | |||
| Smoking frequency in preceding 30 days | |||||
| None (score=0) | 43 | 0 | |||
| Less than 1 cigarette per week (score=1) | 0 | 17 | |||
| Less than 1 cigarette per day (score=2) | 0 | 10 | |||
| 1–5 cigarettes per day (score=3) | 0 | 9 | |||
| 6–10 cigarettes per day (score=4) | 0 | 4 | |||
| 11–20 cigarettes per day (score=5) | 0 | 2 | |||
| More than 20 cigarettes per day (score=6) | 0 | 1 | |||
| Number of lifetime occasions of cannabis use | |||||
| Never (score=0) | 42 | 29 | |||
| 1–2 (score=1) | 1 | 4 | |||
| 3–5 (score=2) | 0 | 4 | |||
| 6–9 (score=3) | 0 | 2 | |||
| 10–19 (score=4) | 0 | 1 | |||
| 20–39 (score=5) | 0 | 1 | |||
| 40 or more (score=6) | 0 | 2 | |||
| Cannabis use frequency in preceding 30 days | |||||
| None (score=0) | 42 | 30 | |||
| 1 or 2 (score=1) | 1 | 6 | |||
| 3–5 (score=2) | 0 | 2 | |||
| 6–9 (score=3) | 0 | 3 | |||
| 10–19 (score=4) | 0 | 0 | |||
| 20–39 (score=5) | 0 | 1 | |||
| 40 or more (score=6) | 0 | 1 | |||
| Mean | SD | Mean | SD | ||
| Score for lifetime smoking | — | 4.53 | 1.70 | ||
| Score for smoking in preceding 30 days | — | 2.23 | 1.32 | ||
| Age at first cigarette (years) | 12.67 | 0.99 | |||
| Age at beginning of daily smoking (years) | 13.35 | 0.74 | |||
| Age at first cannabis use (years) | 15a | 13.43 | 0.94 | ||
| Score on Fagerström Test for Nicotine Dependence | 2.12b | — | |||
| Characteristic | Comparison Subjects (N=43) | Smokers (N=43) | Difference Between Groups | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | pa | |
| Temperament and Character Inventory (15) scores | |||||
| Novelty seeking (total) | 108.6 | 12.04 | 121.98 | 10.16 | <0.000001b |
| Subscales | |||||
| Exploratory excitability | 33.20 | 4.02 | 34.28 | 4.06 | 0.22 |
| Impulsivity | 26.09 | 4.23 | 30.00 | 4.10 | <0.00001b |
| Extravagance | 27.34 | 5.47 | 33.02 | 4.67 | <0.00001b |
| Disorderliness | 21.98 | 4.26 | 24.67 | 3.20 | 0.002b |
| Substance Use Risk Profile Scale (16) scores | |||||
| Anxiety sensitivity | 11.28 | 2.32 | 11.02 | 1.90 | 0.58 |
| Negative thinking | 13.42 | 2.50 | 14.56 | 2.63 | 0.05 |
| Impulsivity | 11.68 | 2.46 | 13.10 | 1.80 | 0.004b |
| Sensation seeking | 13.58 | 3.00 | 13.72 | 2.75 | 0.82 |
| Alcohol Use Disorders Identification Test (17) total score | 1.34 | 2.34 | 6.00 | 3.98 | <0.00001b |
| Delay discount rate (21) (log transformed) | –2.07 | 0.69 | –1.74 | 0.44 | 0.02b |
| Wechsler Intelligence Scale for Children (WISC-IV) (20) | |||||
| IQ estimate (average of subscale scores) | 40.01 | 7.97 | 37.88 | 7.78 | 0.42 |
| Subscale scores | |||||
| Similarities | 31.05 | 7.15 | 29.19 | 7.18 | 0.23 |
| Vocabulary | 51.28 | 11.25 | 49.37 | 10.96 | 0.43 |
| Block design | 50.47 | 13.28 | 57.37 | 12.01 | 0.26 |
| Matrix reasoning | 27.26 | 5.48 | 25.60 | 5.48 | 0.17 |
| N | N | ||||
| Development and Well-Being Assessment (14) estimated probability of any psychiatric disorder | |||||
| None | 6 | 10 | |||
| ∼0.2% | 35 | 21 | |||
| ∼2% | 0 | 4 | |||
| ∼20% | 2 | 8 | |||
| ∼75% | 0 | 0 | |||
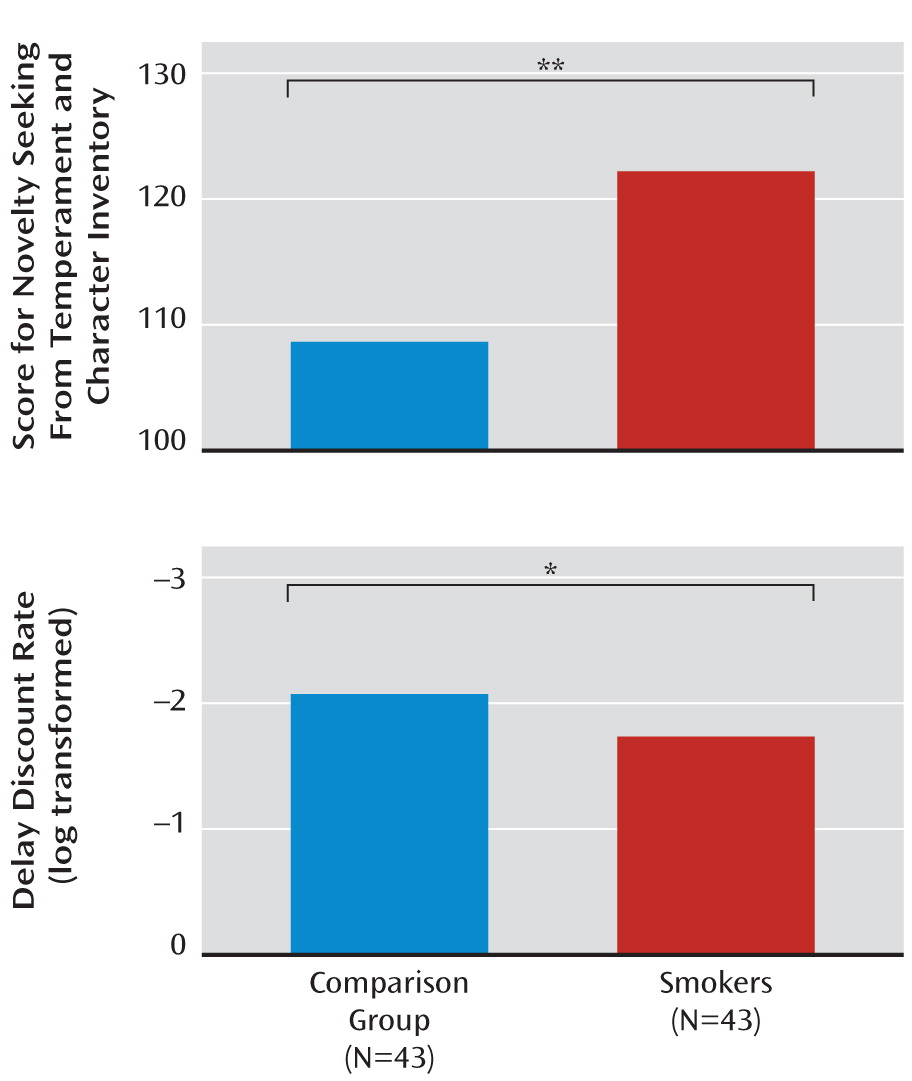
Behavioral Data
| Comparison Subjects (N=43)b | Smokers (N=43)b | Two-Sample t Test, Two-Tailed (p) | |||
|---|---|---|---|---|---|
| Performance Variable | Mean | SD | Mean | SD | |
| Percentage of hits | |||||
| Large reward | 70.09 | 9.04 | 67.86 | 7.84 | 0.10 |
| Small reward | 69.56 | 9.01 | 68.39 | 9.56 | 0.57 |
| No reward | 52.22 | 13.32 | 50.95 | 16.04 | 0.70 |
| Reaction time (msec) | |||||
| Hits | |||||
| Large reward | 226.3 | 25.0 | 241.1 | 24.7 | 0.02 |
| Small reward | 228.8 | 25.9 | 243.4 | 24.1 | 0.01 |
| No reward | 234.7 | 29.5 | 247.7 | 24.0 | 0.04 |
| Misses | |||||
| Large reward | 283.0 | 69.1 | 318.1 | 71.8 | 0.04 |
| Small reward | 296.9 | 74.0 | 319.6 | 65.1 | 0.17 |
| No reward | 327.6 | 78.3 | 350.3 | 54.0 | 0.16 |
fMRI Data
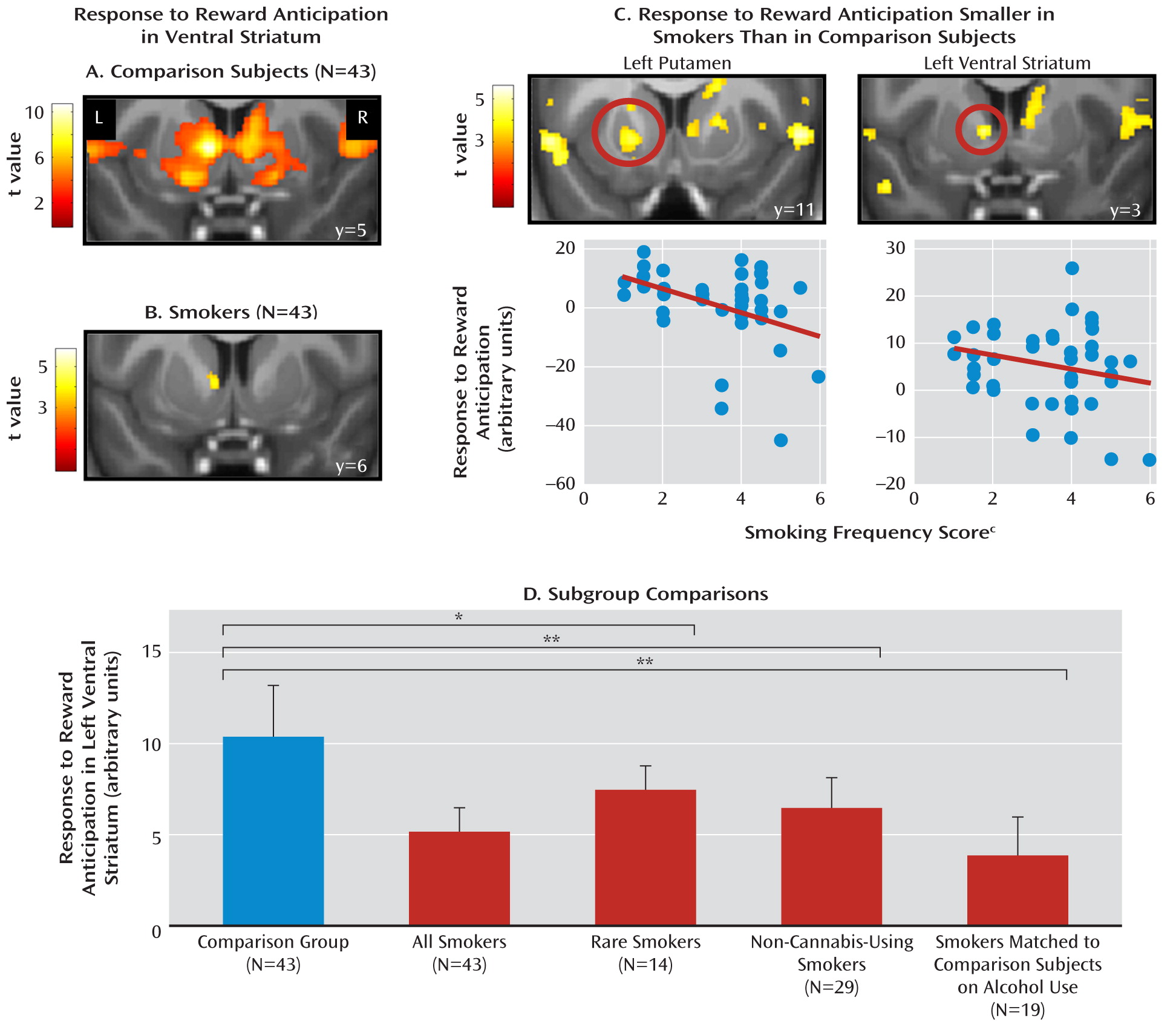
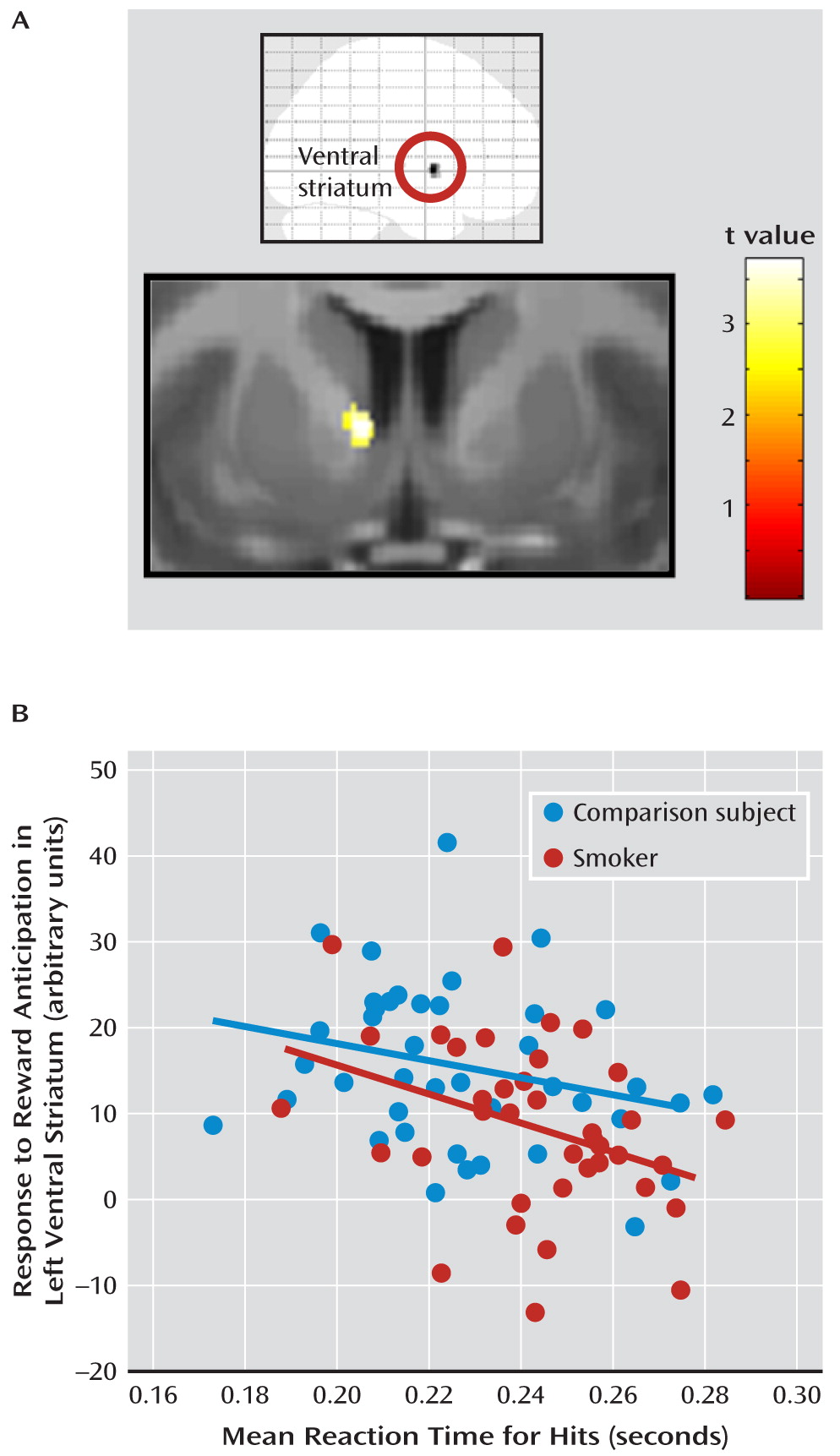
Brain-Behavior Correlations
Discussion
Footnote
Supplementary Material
- View/Download
- 70.57 KB
References
Information & Authors
Information
Published In
History
Authors
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

