Of the emerging copy number variations being identified as rare causes of schizophrenia, hemizygous 22q11.2 deletions are the genetic variants with the highest penetrance that convey the greatest elevation of risk for schizophrenia, estimated to be more than 20 times that of the general population (
1,
2). Approximately one of every 100 patients with schizophrenia in the general population has a 22q11.2 deletion (
3,
4). The associated 22q11.2 deletion syndrome, previously known as velocardiofacial or DiGeorge syndrome (Mendelian Inheritance of Man #188400, #192430), displays a variable phenotype with common features, including velopharyngeal insufficiency, congenital heart disease, and learning difficulties (
5,
6). Mental retardation (IQ <70) is present in a minority of those with the syndrome (
5,
7,
8). The 22q11.2 deletion syndrome has been proposed as a neurodevelopmental model of schizophrenia with enhanced genetic homogeneity that may help us understand the pathogenesis of schizophrenia, including changes in brain structure (
9–
12).
Discussion
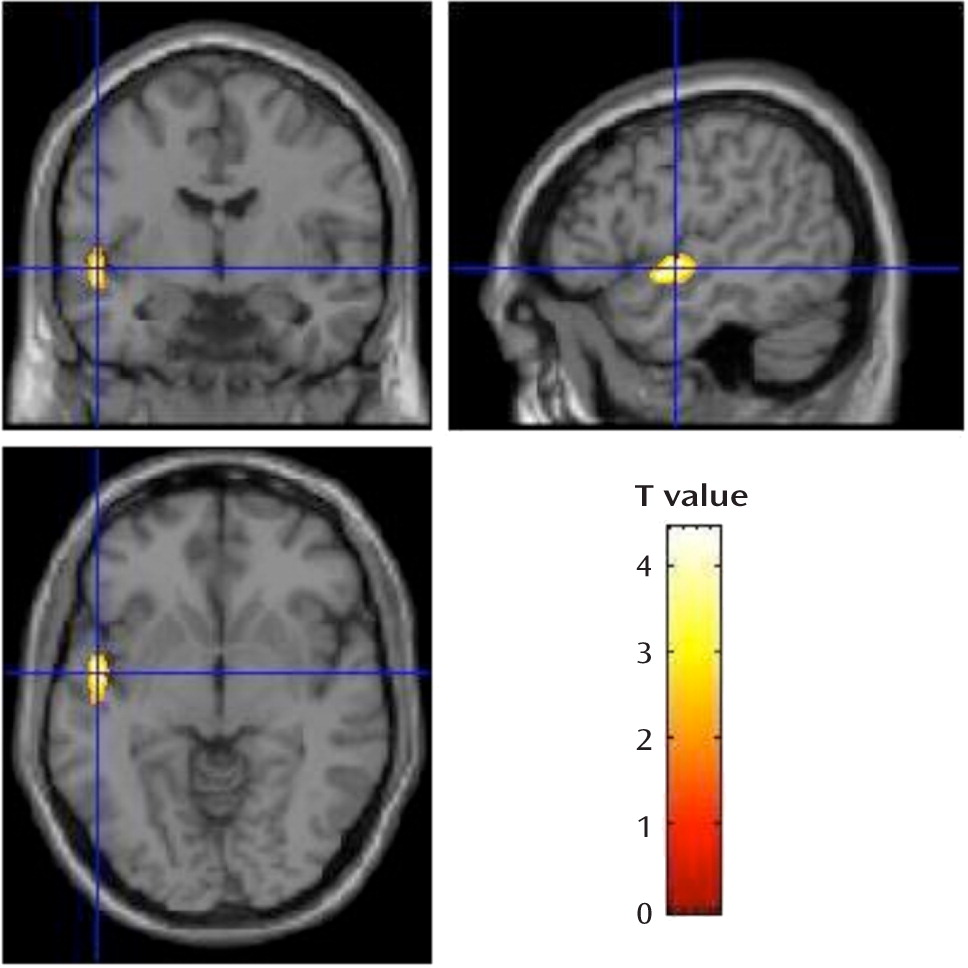
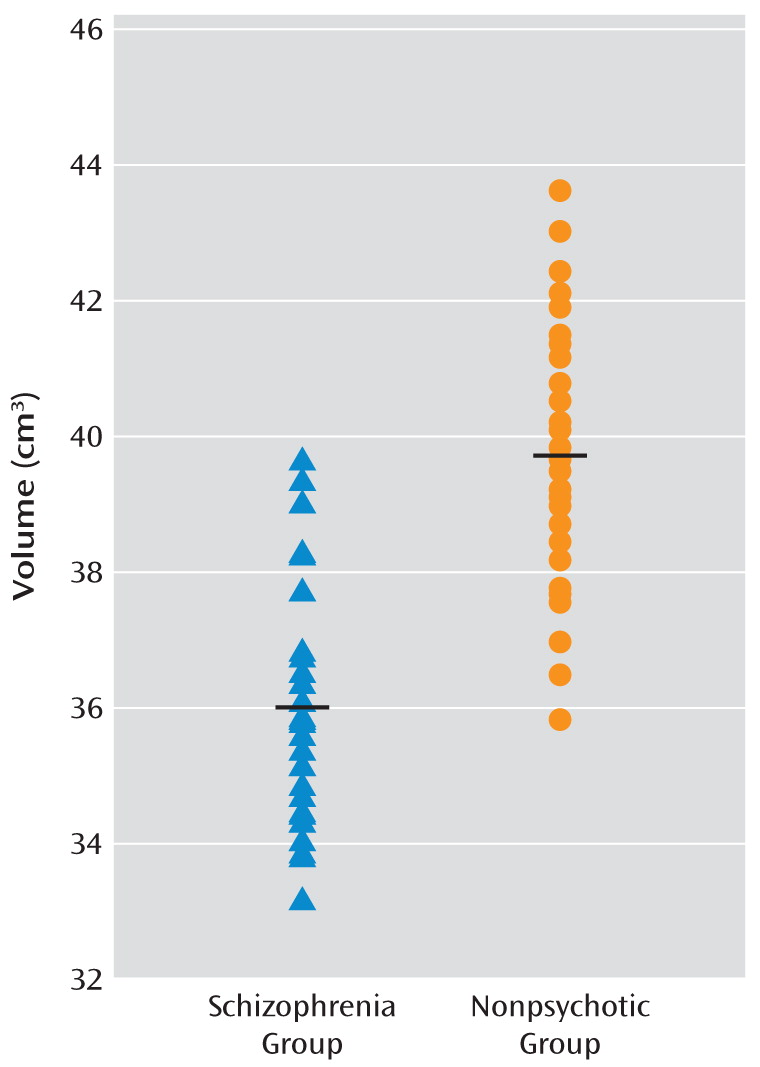
This is the largest brain imaging study of adults with 22q11.2 deletion syndrome to date. Our goal was to determine brain structural changes associated with expression of schizophrenia in this relatively homogeneous group in which all participants carried a hemizygous 22q11.2 deletion. Taken together, voxel-based morphometry and region-of-interest analyses revealed bilateral gray matter volumetric deficits in the temporal lobes, and specifically in the superior temporal gyri, to be significantly associated with the expression of schizophrenia in adults with a 22q11.2 deletion. These findings are consistent with the most highly replicated structural imaging findings in general population samples of schizophrenia patients compared with healthy individuals (
15). Gray matter volumetric deficits in the superior temporal gyrus and related temporal lobe regions have been found before, at, and after onset of psychosis, pointing to this brain region as one of those most prominently and specifically involved in the pathogenesis of schizophrenia (
15–
17,
31–
34). In this study, we found no evidence that other gray matter regions, white matter, or CSF volumetric changes were significantly associated with expression of the schizophrenia phenotype in 22q11.2 deletion syndrome.
Three previous brain imaging studies of adults with 22q11.2 deletion syndrome and schizophrenia have been reported (
10,
13,
14), with sample sizes of 14, 11, and 6, respectively. Only the latter two studies compared schizophrenia patients and individuals with 22q11.2 deletions with no psychotic illness (
13,
14). The small sample sizes and other methodological issues likely limited the ability of those studies to identify consistent or specific MRI brain differences in 22q11.2 deletion syndrome between those with and without schizophrenia. However, consistent with the study by van Amelsvoort et al. (
13), in which the participants were comparable in age to those in our study, we found no significant differences in total gray matter, white matter, and CSF volumes between the groups with and without schizophrenia. Consistent with Schaer et al. (
14), we also found gray matter anomalies in the left superior temporal gyrus. As expected, our findings also differ from those of studies using different study designs. These include the more widespread MRI findings of studies that compare patients with 22q11.2 deletion syndrome and schizophrenia with individuals who have neither 22q11.2 deletion syndrome nor schizophrenia (
10,
13). The differences in findings may be related to the inherent limitations in comparing a relatively homogeneous group with a specific genetic predisposition (hemizygous 22q11.2 deletion) with heterogeneous comparison samples that may have many genetic and other differences in addition to the expression of schizophrenia. This is the case even if IQ is comparably low in the comparison group (
13,
14), where learning difficulties could be attributable to various causes, including other undiagnosed genetic conditions having variable predisposition to schizophrenia.
Our results may also be consistent with those of Bearden et al. (
35), who found evidence of temporal lobe cortical thinning in children with 22q11.2 deletion syndrome relative to comparison children, suggesting that there may be temporal gray matter volumetric or other structural changes in some or perhaps many individuals in the sample at risk for developing schizophrenia. This raises the possibility that, as in the general population and in high-risk familial schizophrenia samples (
34,
36–
38), individuals with 22q11.2 deletions may exhibit brain structural changes at a young age that are similar to those associated with expression of the full disease of schizophrenia later on in life. While a study of 19 adolescents with 22q11.2 deletion syndrome (
39) revealed no significant differences in brain volume over 5 years when comparing the seven participants who developed psychosis with the 12 who did not, the small sample size and the young age might have limited the study's power to detect structural and developmental brain differences associated with schizophrenia. Brain imaging results for adults with 22q11.2 deletion syndrome would be expected to be different from those involving children and adolescents with 22q11.2 deletion syndrome. Studies of children must take into account the brain changes associated with development, which are known to be delayed in this condition, and with the diagnostic uncertainties given an evolving phenotype (
12,
14). Prospective studies of large samples of individuals with 22q11.2 deletion syndrome followed well into adulthood will be needed to further investigate this important issue.
In the present study, comparing adults with 22q11.2 deletion syndrome with and without schizophrenia likely minimized sample-related variability and maximized the likelihood that the differences identified are attributable to expression of schizophrenia and not to other associated features of the syndrome. Our results suggest that studying the gray matter volumetric changes in the superior temporal gyrus and related temporal lobe regions will be important in understanding the pathogenesis of schizophrenia in 22q11.2 deletion syndrome, as for schizophrenia in the general population (
15,
16,
34,
40,
41). Also consistent with several studies of general population samples of schizophrenia patients, we found no significant effect of duration of illness (
16,
42,
43), antipsychotic dosage, or positive symptom severity (
16) on the extent of gray matter volume regional losses. Longitudinal studies, particularly in the years around onset of psychosis, would be needed to investigate the issues of timing, extent of active pathogenic changes (
34), and potential amelioration with antipsychotic medication (
44). However, our results suggest that the main findings are more relevant to expression of schizophrenia per se than to chronicity or severity of illness.
Our sample size was adequate to withstand the well-recognized variability of expression within 22q11.2 deletion syndrome (
7,
10). The mean age of the sample enhances the likelihood that diagnostic classification was stable. If some nonpsychotic individuals with 22q11.2 deletion syndrome subsequently develop schizophrenia, such diagnostic misclassification would serve to make it more difficult to detect true differences between the groups. We follow these patients longitudinally, however, minimizing this possibility. Notably, our methods accounted for IQ, presence of major congenital cardiac disease, and total intracranial volume, which are all issues of concern in studying the expression of schizophrenia in 22q11.2 deletion syndrome (
12). To further evaluate the finding of reduced superior temporal gyrus volume in schizophrenia in our whole-brain voxel-based morphometry analyses, we analyzed region-of-interest volumes. These analyses might have allowed for more sensitive identification of bilateral regional findings than using the whole-brain approach, where the greater number of multiple comparisons were more strictly corrected for by the false recovery rate.
Our analysis also had limitations typical of cross-sectional and voxel-based morphometry studies. These include the inability to detect ventricular enlargement or increased CSF volume that may be associated with schizophrenia. However, such changes may be less specific; for example, they may be related more to cognitive variables in 22q11.2 deletion syndrome (
12,
35). Developmental anomalies such as neuronal migration abnormalities and midline defects also may be associated with expression of schizophrenia in 22q11.2 deletion syndrome (
45,
46). These would not be observable with voxel-based morphometry, although they may be compatible with the gray matter volumetric deficits observed using this imaging method. Higher-resolution scanning and other analytic methods may be needed to reveal other relevant structural anomalies associated with schizophrenia in this syndrome.
Difficulties in 22q11.2 deletion syndrome sample collection, such as pacemakers or claustrophobia precluding MRI scanning, are well known. While this is the largest MRI study of adults with 22q11.2 deletion syndrome to date, larger samples could increase the statistical power to show additional significant findings of smaller effect size than those observed. These may or may not include the frontal lobe gray matter volumetric findings seen in comparisons between adults with 22q11.2 deletion syndrome and comparison samples (
10,
13) or other temporal lobe or subcortical gray matter volumetric changes seen in other genetic subtypes of schizophrenia (
36).
Remaining questions of interest include why a minority of individuals with 22q11.2 deletion syndrome develop schizophrenia while the majority do not and what light these associated structural brain findings may shed on pathogenetic mechanisms. The mechanism underlying the variable expression associated with deletion 22q11.2 syndrome, even in mouse models, remains a mystery. We previously reported (
18) that there were no significant effects of length of the 22q11.2 deletion, parental origin of the 22q11.2 deletion, parental age, or family history on expression of schizophrenia in 100 adults with 22q11.2 deletion syndrome. While hemizygosity of the approximately 45 genes in the commonly deleted 22q11.2 region seems to confer the major copy number-related risk factor for expression of schizophrenia, other factors may modulate this risk (
3,
18). Murine models suggest that multiple gene dosage effects may play a role (
47,
48). Other possibilities include altered expression related to genetic variants within the intact 22q11.2 region or in the rest of the genome (
18). However, there is no evidence that the COMT functional allele influences expression of schizophrenia in 22q11.2 deletion syndrome (
11,
21), and results for other genes in the 22q11.2 deletion region are inconclusive. Interacting environmental effects also deserve consideration (
49). Cumulative effects of the hemizygous 22q11.2 deletion, other genetic variants, or other factors could affect the changes in neuronal cell migration, synaptogenesis, synaptic plasticity, or neurogenesis that represent plausible mechanisms for schizophrenia. Aberrant brain maturation and neuronal migration anomalies are well documented in 22q11 deletion syndrome (
46,
50). Gray matter deficits in the superior temporal gyrus suggest that, as in other forms of schizophrenia, primary sensory processing cortex and cortical regions that are specialized for language and speech processes may be involved (
16,
37). There is initial evidence that glutamate synapses in this region may be implicated (
51).