Categorization of serious mental illness remains controversial, with current diagnoses having vague boundaries, weak diagnostic validity, and limited promise for biological significance (
1). Current diagnostic categories (e.g., schizophrenia and bipolar disorder) are based predominantly on phenomenological criteria and are not supported by biological definitions. The identification of pathophysiologically relevant disease biomarkers and testing these biomarkers as novel targets for treatment are critical for the field. The Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) is a multisite research collaboration established to test the manifestations and distribution of intermediate phenotypes across schizophrenia-bipolar diagnostic categories and to examine genetic associations of these intermediate phenotypes (
2). Given the substantial overlap in clinical, neurophysiological, and molecular characteristics of schizophrenia and bipolar disorder (
1), the B-SNIP has focused on testing a dimensional organization of psychosis, a well-defined clinical phenotype that can potentially serve as a useful dimension for exploring underlying disease biomarkers. Testing an intermediate phenotype dimensionally includes pooling all probands with the targeted clinical manifestation (e.g., psychosis) regardless of diagnoses, organizing their biological relatives by the phenotype expression into affected and unaffected, and contrasting all groups with healthy individuals. This approach was undertaken here to test whether psychosis-centered categorization of serious mental illness across schizophrenia-psychotic bipolar I disorder diagnoses would generate groups of probands and relatives with more homogenous disease biomarkers, as measured by whole brain gray matter voxel-based morphometry.
Considerable evidence from previous structural MRI reports supports robust reductions in gray matter volume and/or density throughout cortical and subcortical structures in probands with schizophrenia, with the most substantial deficits in the frontotemporal regions (
3–
5). In bipolar probands, recent meta-analyses have described gray matter reductions in the prefrontal, anterior cingulate, insular, and temporal cortices, overlapping with those observed in schizophrenia, albeit less extensively (
6–
8). Small studies focusing on gray matter volume in psychotic bipolar disorder have reported variable results, ranging from schizophrenia-like gray matter reductions (
9) to near-normal neocortical volumes (
10,
11). Even more controversy surrounds the conceptualization of schizoaffective disorder as a distinct diagnostic entity (
12); schizoaffective probands are often intermingled with schizophrenia case subjects and show similar gray matter characteristics (
13,
14).
Studies of biological relatives of psychosis probands have found variable gray matter alterations in the basal ganglia, parahippocampal gyrus, and prefrontal cortex in the relatives of schizophrenia probands (
15) and in the frontotemporal regions in relatives of bipolar probands (
16). Clinical heterogeneity within the relative samples may contribute, with various subgroups of relatives (e.g., affected compared with unaffected relatives) showing distinctive gray matter phenotypes. Characteristically, psychosis spectrum disorders (e.g., schizotypy) have been linked to decreased gray matter volumes in the frontotemporal, parietal, and insular cortices (
17–
19), whereas no such abnormalities have been observed in unaffected relatives (
20). Overall, these findings suggest that probands across schizophrenia-bipolar diagnoses have regionally overlapping gray matter volume reductions (commonly in the frontotemporal neocortex and more extensively in schizophrenia), although reports in bipolar disorder are highly variable. Little is known specifically about gray matter phenotypes in psychotic bipolar disorder. While findings in relatives are inconsistent, relatives with psychosis spectrum disorders tend to show gray matter reductions similar to psychosis probands, suggesting heritability of these structural alterations and their linkage to psychosis. Few studies have contrasted volumetric changes across proband and relative groups defined by the psychosis dimension (
20).
We examined gray matter volumetric phenotypes within the schizophrenia-psychotic bipolar disorder spectrum contrasted by the psychosis dimension or DSM-IV diagnoses. The psychosis dimension was defined by lifetime axis I (in probands) or axis II (cluster A, in relatives) psychosis spectrum diagnoses; the probands as a whole, and relatives with and without cluster A or psychosis spectrum disorders composed the psychosis dimension groups. We tested whether a common gray matter phenotype manifests in probands and relatives across the psychosis dimension and whether gray matter characteristics would discriminate DSM-IV diagnostic groups. Therefore, two sets of analyses were conducted: one contrasting gray matter volumes in probands and relatives across the psychosis dimension and the other contrasting probands and relatives within categorical DSM-IV diagnoses (see Statistical Analyses for details). We hypothesized that 1) across the psychosis dimension, psychosis probands would show reduced gray matter volumes in numerous cortical and subcortical regions, relatives with psychosis spectrum disorders would show milder gray matter deficits regionally overlapping with those in probands, and nonpsychotic relatives would show normal gray matter volumes relative to healthy comparison subjects; and 2) across DSM-IV diagnoses, probands with schizophrenia, probands with schizoaffective disorder, and probands with psychotic bipolar I disorder would show regionally overlapping gray matter reductions that were most extensive in the frontotemporal cortex, with their magnitude being largest in schizophrenia and smallest in bipolar probands, and that their relatives would show overlapping frontotemporal gray matter changes intermediate in magnitude between probands and healthy comparison subjects.
Method
Study Sample
In total, 920 case subjects, including 351 psychosis probands (146 with schizophrenia, 90 with schizoaffective disorder, and 115 with psychotic bipolar I disorder), 369 relatives (134 were relatives of individuals with schizophrenia, 106 of individuals with schizoaffective disorder, and 129 of individuals with psychotic bipolar I disorder), and 200 healthy comparison subjects were recruited into the same dense phenotyping protocol across four B-SNIP sites between January 2008 and February 2011. Detailed characteristics of the B-SNIP clinical population are described elsewhere (
2). All case subjects provided written informed consent after the study procedures had been fully explained. The axis I diagnoses for the probands and relatives were based on the Structured Clinical Interview for DSM-IV Axis I Disorders/Patient Edition (SCID-I/P) (
21), and relatives’ axis II diagnoses were based on the Structured Interview for DSM-IV Personality (SIDP-IV) (
22) (
Figure 1). Probands were stable medicated outpatients; relatives with lifetime psychiatric diagnoses were asymptomatic or mildly symptomatic at the time of the imaging acquisition. Relatives who met the criteria for axis I psychotic disorders (41/369 with proband-like psychosis diagnoses [schizophrenia, schizoaffective disorder, or psychotic bipolar I disorder] and 8/369 with other axis I psychoses) were excluded from these analyses. In all, 34 relatives met DSM-IV or SIDP-IV criteria for a cluster A or psychosis spectrum personality disorder and were included. The nonpsychotic relatives group included relatives with no lifetime psychiatric diagnoses (completely unaffected) and those with nonpsychotic axis I or axis II diagnoses (e.g., mood and anxiety disorders or cluster B and C personality disorders). Rates of DSM-IV axis I or axis II diagnoses in relatives are presented in the
data supplement that accompanies the online edition of this article.
The demographic and clinical characteristics of the study sample are outlined in
Table 1. Between-group differences were observed in age, accounted for by the older age of relatives compared with probands. Schizophrenia probands had a higher proportion of male individuals compared with other groups. Between-group differences were also found in handedness as a result of a higher number of ambidextrous individuals among the schizoaffective relatives compared with the rest of the relative groups and the healthy comparison subjects. While groups did not differ in ethnicity, differences were found in race, with a higher proportion of African Americans among schizophrenia and schizoaffective probands and schizophrenia relatives compared with bipolar probands and healthy comparison subjects. Between-group differences were also found in years of education, where schizophrenia and schizoaffective probands had lower education attainment than all other groups.
The reading subtest scores from Wide-Range Achievement Test (WRAT), used as an estimate of premorbid intellectual functioning, differed across groups, with lower scores in schizophrenia and schizoaffective probands compared with bipolar probands, relatives of schizoaffective and bipolar probands, and comparison subjects. The proband groups did not differ in age at illness onset, age at first psychiatric hospitalization, or lifetime number of hospitalizations. Schizophrenia and schizoaffective probands had higher Positive and Negative Syndrome Scale (PANSS) (
23) total scores and positive and negative subscales scores compared with bipolar probands; schizoaffective probands had higher PANSS general subscale scores compared with the rest of proband groups. In addition, schizoaffective probands had higher Montgomery-Åsberg Depression Rating Scale (MADRS) (
24) scores compared with schizophrenia and bipolar probands, whereas no between-group differences in Young Mania Rating Scale (
25) scores were found. All proband and relative groups scored lower than comparison subjects on the Global Assessment of Functioning, with the lowest scores found in schizophrenia and schizoaffective probands.
Most probands (318/351, 90.6%) were actively treated with various psychotropic medications, including antipsychotics (82.3%), mood stabilizers (49.9%), and antidepressants (44.2%); 253 probands (72.1%) were receiving more than one psychotropic agent. Among relatives, many were unmedicated; antidepressants were the most common medication in the relative groups.
MRI Acquisition and Voxel-Based Morphometry Procedures
Whole-brain structural MRI three-dimensional acquisitions were performed on 3-T scanners (GE Signa, Philips Achieva, Siemens Allegra, and Siemens Trio). All participants at each site were scanned on the same magnet. High resolution isotropic T
1-weighted MP-RAGE sequences were obtained following the Alzheimer’s Disease Neuroimaging Initiative (ADNI) protocol (
http://www.loni.ucla.edu/ADNI/Research/Cores/). The MP-RAGE sequence parameters were comparable across sites (see details in the online
data supplement).
All images were processed by experienced analysts (image preprocessing by ASB and data analyses by EII) who were blind to the participants’ clinical characteristics. T
1-weighted MP-RAGE images were prepared for voxel-based morphometry analyses in SPM8/VBM8/MATLAB7 following standardized steps (
26): reorientation, high-dimensional nonlinear diffeomorphic anatomical registration using the modulation tool (DARTEL) segmentation and normalization that incorporates precise correction for individual brain size (
27), and smoothing. Segmented images were modulated or scaled by the amount of warping to maintain the total amount of gray matter volume (
28). Modulated gray matter images were smoothed with a 12-mm isotropic Gaussian kernel and selected for the group-level statistical analyses.
Statistical Analyses
A one-way analysis of variance (ANOVA) with a subsequent post hoc Tukey honestly significant difference test and Yates corrected chi-square test were used, as appropriate, for demographic and clinical variables. To test a priori hypotheses, the primary analyses contrasted gray matter volumes across the psychosis dimension groups (probands, relatives with psychosis spectrum disorders, nonpsychotic relatives, and healthy comparison subjects) and DSM-IV diagnostic groups (schizophrenia, schizoaffective, and psychotic bipolar I probands, their respective relatives, and healthy comparison subjects). These analyses were completed using SPM8 full factorial design. Absolute threshold masking was set at 0.1. All analyses were adjusted for individual brain volume during DARTEL segmentation/normalization step. The distinctive group demographic characteristics that are known to affect brain structure (age, sex, and handedness) (
29,
30) as well as site were included as covariates in all statistical models. In addition, all analyses comparing related individuals (i.e., probands and relatives from the same pedigree) were adjusted for random family effects. No diagnosis-by-site interaction (healthy comparison subjects and the three proband groups × four sites) was observed, even at the least stringent threshold (p<0.05, false discovery rate corrected). In addition, between-site comparisons for the healthy comparison subjects and schizophrenia probands revealed similar regional distributions of gray matter reductions across sites, further supporting between-site data comparability (see Figure S1 in the online
data supplement). All primary voxel-based morphometry outcomes are reported at a p<0.005, false discovery rate corrected, k=200 contiguous voxels threshold, providing the most informative between-group volumetric differences (see Figure S2 in the online
data supplement). Regional outputs were identified using the Group ICA for fMRI Toolbox, GIFT1.3i (
31;
www.sourceforge.net) checked against a standardized anatomical brain atlas (
32).
In addition, exploratory multiple regression analyses were carried out to examine associations between gray matter volumes and symptom severity (using PANSS, Young Mania Rating Scale, and MADRS scores) and illness duration (adjusted for age, gender, and site) in the proband groups. Associations between gray matter volume and concomitant medication use (use status dichotomized as on or off antipsychotics, mood stabilizers, and antidepressants) were computed in all probands combined (given the small number of participants treated with mood stabilizers and antidepressants) as well as in the bipolar probands alone for current lithium use (i.e., multiple regression analysis with status dichotomized as on or off lithium and direct comparisons of gray matter volume in bipolar probands on [N=26] or off [N=26] lithium). Because the number of relatives on medications was low, especially for antipsychotics and mood stabilizers, we only computed correlations between gray matter volumes and antidepressant use in all relatives combined. All exploratory outcomes were tested at both p<0.005 and p<0.05, false discovery rate corrected, k=200 contiguous voxels thresholds.
Results
Gray Matter Volume Phenotypes Across the Psychosis Dimension
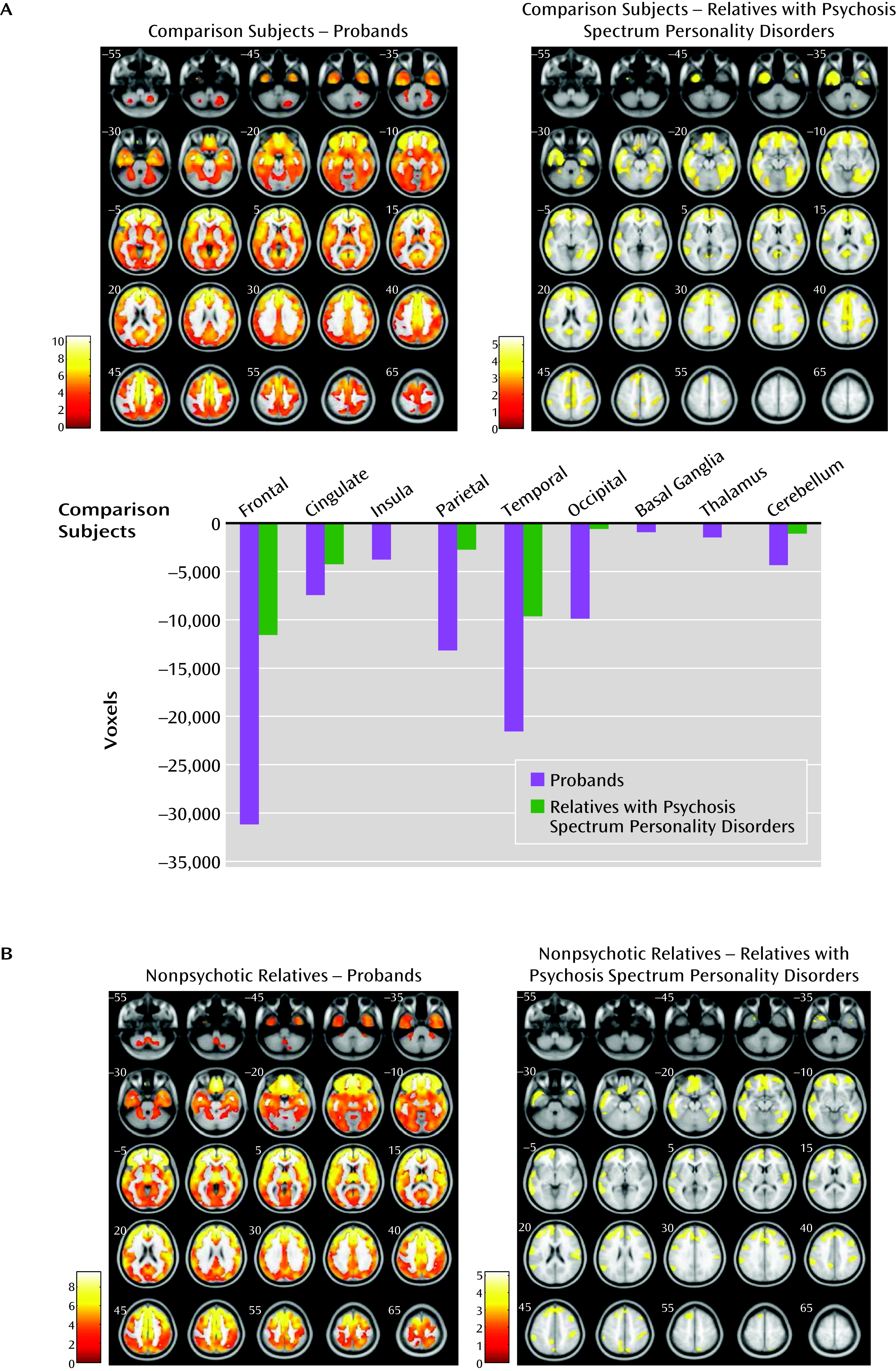
Regional gray matter volumes contrasted along the psychosis dimension (probands, relatives with psychosis spectrum disorders, nonpsychotic relatives, and healthy comparison subjects) revealed a between-group effect (F=2.98, df=5, 903, p<0.005, false discovery rate corrected, k=200) (
Figure 2A and see Table S2 in the online
data supplement). Subsequent pairwise comparisons revealed diffuse gray matter volume reductions in psychosis probands relative to healthy comparison subjects in the frontal, anterior/posterior cingulate, insular, temporal, parietal, and occipital cortices and in the basal ganglia, thalamus, and cerebellum. Relatives with psychosis spectrum disorders contrasted with healthy comparison subjects showed volume reductions regionally overlapping those found in probands, albeit less extensive. No differences emerged between nonpsychotic relatives and comparison subjects. No differences were observed between the two subgroups within the nonpsychotic relatives group (i.e., completely unaffected and those with nonpsychotic axis I or axis II diagnoses) and healthy comparison subjects. No gray matter volume increases were observed in any psychosis dimension group relative to healthy comparison subjects.
Pairwise comparisons between the psychosis dimension groups revealed overlapping gray matter volume reductions in the frontotemporal, anterior cingulate, and parietal regions in the psychosis probands and in relatives with psychosis spectrum disorders compared with nonpsychotic relatives (
Figure 2B and see Table S2 in the online
data supplement). No differences were found between probands and relatives with psychosis spectrum disorders. No differences emerged between completely unaffected relatives compared with relatives with nonpsychotic diagnoses within the nonpsychotic relatives group. No volume increases were found in either the psychosis probands or relatives with psychosis spectrum disorders compared with nonpsychotic relatives.
Gray Matter Volume Phenotypes Across DSM-IV Diagnoses
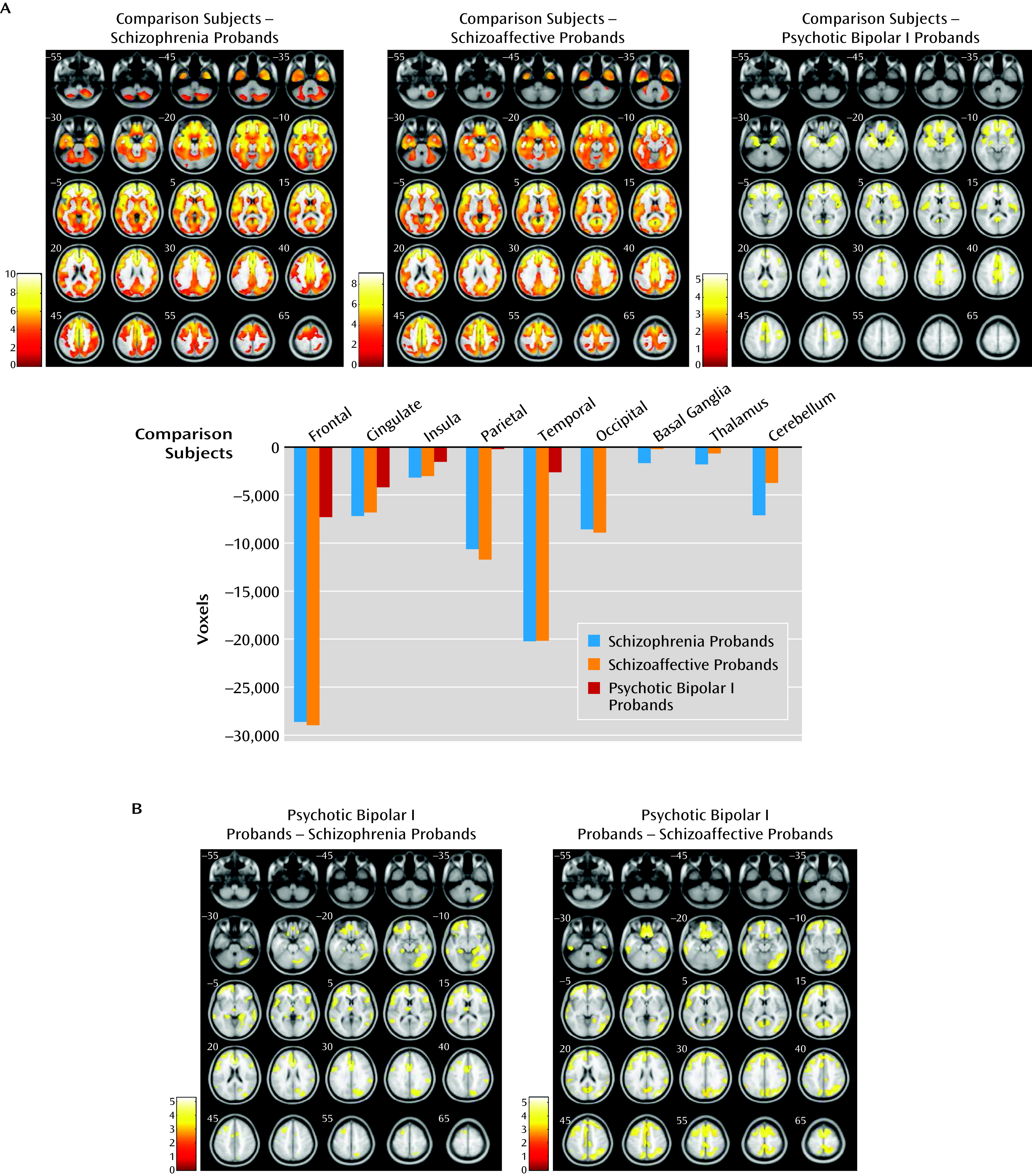
The proband group was divided by diagnosis (schizophrenia, schizoaffective disorder, and psychotic bipolar I disorder) and assessed against healthy comparison subjects, and a significant between-group effect was observed (F=3.9, df=3, 542, p<0.005, false discovery rate corrected, k=200). Subsequent pairwise comparisons revealed substantial overlapping gray matter reductions in schizophrenia and schizoaffective probands in the frontal, anterior/posterior cingulate, insular, temporal, parietal, and occipital cortices, as well as in the basal ganglia, thalamus, and cerebellum relative to healthy comparison subjects. Probands with psychotic bipolar disorder showed smaller clusters of gray matter reduction in the frontal, anterior/posterior cingulate, insular, temporal, and parietal cortices relative to healthy comparisons subjects, regionally overlapping with those in schizophrenia and schizoaffective probands (
Figure 3A and see Table S3 in the online
data supplement). No increases in gray matter volume were found in any proband group relative to healthy comparison subjects.
Pairwise gray matter volume contrasts between proband groups revealed no differences in schizophrenia probands compared with schizoaffective probands in any brain regions (
Figure 3B and see Table S3 in the online
data supplement). Both schizophrenia and schizoaffective probands had reduced gray matter volumes compared with psychotic bipolar I probands throughout the neocortex and cerebellum; schizophrenia probands showed an additional cluster of gray matter reduction in the left thalamus compared with bipolar probands. No gray matter volume increases characterized either schizophrenia or schizoaffective probands compared with bipolar probands.
The relatives contrast revealed no effect of diagnostic group (schizophrenia, schizoaffective disorder, bipolar disorder, and healthy comparison subject groups) even at the least stringent threshold (p<0.05, false discovery rate corrected).
Associations Between Gray Matter Volume and Lifetime Psychosis Duration, Active Symptoms Severity, and Medication
In probands with schizoaffective disorder, lifetime duration of psychosis correlated inversely with gray matter volumes in the basal ganglia bilaterally, the right temporal lobe, and the thalamus (p=0.05) (see Table S4 in the online
data supplement). No significant correlations between gray matter and psychosis duration were found in either schizophrenia or bipolar probands.
Furthermore, in schizoaffective probands, PANSS positive subscale scores correlated inversely with gray matter volumes throughout the neocortex (p=0.005). In schizophrenia probands, PANSS positive subscale scores correlated inversely with gray matter volumes in the right insular and the left parahippocampal gyrus (p=0.05) (see Table S4 in the online
data supplement). No other significant correlations emerged between gray matter volume and PANSS, MADRS, or Young Mania Rating Scale scores in any proband group.
No associations between gray matter volume and current use of antipsychotics, mood stabilizers, or antidepressants were found among probands. No association between gray matter volume and active lithium treatment was evident in probands with psychotic bipolar I disorder. Likewise, no between-group differences in gray matter volume were found in bipolar probands on lithium compared with off lithium. No relationship between active antidepressant use and gray matter volume was found in relatives. All exploratory medication outcomes were nonsignificant at p=0.05, false discovery rate corrected, k=200 threshold.
Discussion
This study presents voxel-based morphometry-based gray matter volumetric outcomes contrasted between the psychosis dimension and DSM-IV categorical diagnoses groups from a large multisite psychosis sample (B-SNIP). The psychosis dimension analysis revealed diffuse gray matter reductions in both the psychosis probands and in relatives with psychosis spectrum disorders in overlapping cortical regions (e.g., the frontotemporal, parietal, cingulate, and insular cortices) and the cerebellum relative to healthy comparison subjects. By contrast, relatives unaffected by psychosis, even those with lifetime nonpsychotic axis I or axis II diagnoses, had normal gray matter volume. The DSM-IV diagnosis analysis in probands relative to healthy comparison subjects revealed extensive gray matter reductions in numerous cortical and subcortical regions that were similarly distributed in schizophrenia and schizoaffective probands. Psychotic bipolar I probands had gray matter volume reductions primarily localized to the frontotemporal, cingulate, and insular cortices that were regionally overlapping with those in schizophrenia and schizoaffective probands, albeit less extensive. Probands with schizophrenia and those with schizoaffective disorder showed smaller cortical and subcortical gray matter volumes compared with bipolar probands. First-degree relatives of the psychosis probands, when categorized by DSM-IV diagnoses, had gray matter volumes indistinguishable from comparison subjects, contrary to our a priori prediction (
Table 2).
Growing evidence suggests that psychosis as a clinical phenotype may have common biological underpinnings across categorical diagnoses (
1). Measurable gray matter changes are observed in individuals near psychosis onset, progress during the initial years of psychosis, and ultimately result in characteristic gray matter alterations found in chronic psychosis samples (
33,
34). Data from relatives with psychosis spectrum disorders demonstrate milder but similar gray matter volume reductions (
17–
19), suggesting a heritable link between psychosis and brain structure phenotypes. Our findings within the psychosis dimension indicate substantial and overlapping gray matter volume reductions in psychosis probands and relatives with psychosis spectrum disorders, in contrast to normal gray matter in nonpsychotic relatives. These gray matter alterations may reflect “psychosis burden,” from extensive reductions in probands where psychosis is fully manifested, to similarly distributed but milder alterations in relatives with mild psychosis spectrum phenotypes, to normal gray matter in relatives without lifetime psychosis spectrum disorders. Remarkably, relatives categorized by traditional schizophrenia or bipolar disorder diagnoses showed no alterations in gray matter volume. Furthermore, a subgroup of relatives with lifetime nonpsychotic axis I or axis II diagnoses had normal gray matter volumes, consistent with previous reports (
20). Our findings from analyses of DSM-IV diagnosis in probands, i.e., robust gray matter reductions in probands with schizophrenia and schizoaffective disorder compared with milder overlapping reductions in probands with psychotic bipolar I disorder, may reflect cumulative lifetime psychosis burden in these psychiatric conditions, with psychosis assumed to be more pervasive in schizophrenia and schizoaffective disorder than in bipolar disorder. This is further supported by inverse correlations between lifetime duration of psychosis and gray matter volume in schizoaffective probands in the basal ganglia, the temporal cortex, and the thalamus, the regions long implicated in psychosis in both first-episode and chronic samples (
3–
5). Inverse correlations between frontotemporal gray matter volumes and PANSS psychosis scores in schizoaffective and schizophrenia probands also support a link between psychosis and gray matter reduction. Overall, these findings suggest that psychosis is a clinical dimension characterized by unique gray matter intermediate phenotypes, namely by reductions primarily localized to the frontotemporal neocortex.
Our findings across categorical diagnoses indicate partially divergent gray matter volume characteristics for schizophrenia and schizoaffective disorder (with extensive cortical and subcortical gray matter reductions) compared with psychotic bipolar I disorder (with limited gray matter reductions in the frontotemporal and parietal cortex) that are different in the extent and magnitude but that overlap in regional distribution, consistent with previous reports (
3–
5,
8). The absence of differences between schizophrenia and schizoaffective case subjects raises questions about the biological uniqueness of the schizoaffective disorder construct (
12), while the substantive differences between schizophrenia/schizoaffective disorder and psychotic bipolar I disorder provide some support for the schizophrenia-bipolar distinction. The mechanisms underlying these gray matter differences are unknown but can be interpreted in the context of published postmortem findings of gray matter pathology in the neocortex. Histological examination of individuals with schizophrenia indicates no neocortical neuronal loss but altered neuronal cell packing, presumably due to reduced interstitial neuropil, resulting in higher neuronal density and decreased cortical thickness (
35). In contrast, analyses from individuals with bipolar disorder reveal decreased neuronal and glial density but normal overall cortical thickness (
35). These distinct postmortem findings in schizophrenia compared with bipolar disorder suggest at least partially unique anatomical underpinnings for the two illnesses, providing plausible cellular correlates for the divergent gray matter findings in schizophrenia/schizoaffective probands and psychotic bipolar probands that were observed here. Further structural MRI analyses parsing cortical thickness and surface area may shed light on this issue. Alternatively, it is possible that a relative gray matter volume preservation in bipolar probands could be secondary to a medication effect (i.e., chronic treatment with lithium) and that even if a primary disease-associated loss of neocortical volume exists in these probands, it could be obscured by a volume-enhancing effect of chronic lithium use (
36,
37). These two explanations could co-occur. No postmortem studies have contrasted psychotic and nonpsychotic variants of bipolar disorder or tissue from individuals with psychosis spectrum disorders, thus leaving open the question of whether the cellular alterations observed in schizophrenia would generalize to the psychosis dimension.
Previous reports provide support for an effect of psychotropic medications on gray matter volume, with increased gray matter volume or density in the basal ganglia associated with first-generation antipsychotics (
38), decreased gray matter volume in the frontotemporal cortex associated with both first- and second-generation antipsychotics (
39,
40), and increased gray matter volume or density in diffuse neocortical regions associated with lithium (
6,
7,
36,
37). The effect of current medication use was tested here, and the results were negative. However, this outcome could be obscured by the high frequency of mixed medication use (61%−83%) in all proband groups, as well as by the longitudinal effects of both disease and medication on brain structure. Given that the majority of probands reported years of chronic treatment with various psychotropic agents, it is possible that the extensive gray matter deficits in probands with schizophrenia and schizoaffective disorder observed here could be, in part, accounted for by the effect of lifetime antipsychotic use, whereas a relative preservation of gray matter volumes in bipolar probands may be related to chronic lithium treatment, despite our negative findings for active medication use. Disentangling a primary disease effect from a medication confound presents considerable difficulty, especially in a cross-sectional study such as ours, and it cannot be accomplished in probands alone. Nevertheless, with the benefit of data from relatives, we suggest that the characteristic gray matter reductions found in relatives with psychosis spectrum disorders where individuals were largely untreated support at least partial independence of these gray matter alterations from medication and suggest their link to psychosis.
Overall, these results from a large sample of psychosis probands and relatives suggest that the dimensional conceptualization of psychosis is a useful approach for investigations targeting biological markers of serious mental illness. The strengths of our study are the relatively large sample, the study of psychosis probands and their relatives across DSM-IV psychosis categories, and the comprehensive clinical characterization of these individuals that allowed a systematic investigation of the psychosis dimension. Limitations include the cross-sectional nature of the study and the potential confounds related to medications and state of illness. The findings generated by the voxel-based morphometry analyses need to be confirmed and expanded by region-of-interest approaches to morphometric quantification. Future research examining the associations between brain structure and other putative intermediate phenotypes, as well as their molecular underpinnings, may aid in the development of a biologically based classification of serious mental illness to leverage discovery and treatment.
Acknowledgments
Gunvant K. Thaker, M.D., was closely involved with the leadership and conceptual and methodological aspects of the study. Dr. Thaker has retired from the research field, and the authors thank his contribution. The authors also thank Bradley Witte, B.S., at UT Southwestern Medical Center and Amy Eliot, M.S., at Maryland Psychiatric Research Center, University of Maryland School of Medicine, for assistance with database management; Dorothy Denton, B.A., at UT Southwestern Medical Center for help with the article preparation; all clinicians for patients’ referral; and, most importantly, the patients and their families who took part in this study.