For almost two decades now, human interferon alpha has been cloned and produced by recombinant DNA technology.
1 Its wide clinical application in the treatment of various neoplastic diseases and viral infections has yielded partial and complete remissions in hematological disorders such as hairy cell leukemia; in chronic myeloid leukemia; in some other chronic myeloproliferative and lymphoproliferative disorders; and in chronic hepatitis.
2–6 Partial success could be achieved in malignant melanoma and hypernephroma,
7–9 and reports on trials in the treatment of AIDS-related Kaposi's sarcoma with recombinant human interferon alpha (rIFN) are promising.
10–12Interferon therapy is known to induce some undesirable side effects.
13,14 A transient flu-like syndrome including fever, chills, fatigue, and muscle pain occurs frequently during the initial treatment phase. In addition, leukopenia, elevated transaminases, hypotension, tachycardia, and neurotoxic symptoms—mainly confusion and somnolence—have been reported. Most of these side effects are strongly dose-dependent and generally prove to be reversible after dose reduction or termination of the treatment.
Only a few studies have specifically dealt with the neurological side effects of rIFN therapy. They either concerned short-term application of high doses
15–17 or reported single observations in patients under low-dose rIFN.
18,19 High doses induced severe neurological side effects—such as paresthesia, the development of an encephalitis-like syndrome, somnolence, lethargy, impaired concentration, and disorientation—and were associated with marked diffuse disturbances in the EEG.
20,21 Treatment with low-dose rIFN resulted in either no neurological side effects at all or rare incidences of lethargy, disorientation, and paresthesia.
21Our investigation pertains to neurological side effects of long-term low-dose rIFN therapy, the most successful rIFN regimen applied in the treatment of hematological neoplasias. We have studied patients with myeloproliferative disorders, afflictions that are known to respond to rIFN therapy
22 and that render the patients susceptible to microcirculatory disturbances. The aim of our study was to evaluate the effects of long-term rIFN therapy on selected parameters of neurological and neuropsychological function.
RESULTS
rIFN dosage started at a median of 25 mU/week (range 10–35) and was steadily decreased to median values of 20 mU/week (range 10–25), 17.5 mU/week (range 10–25), 15 mU/week (range 6–25), and 12 mU/week (range 6–25) at months 3, 6, 9, and 12, respectively. Normalization of platelet counts was achieved by 12 patients after a median time of 7.5 weeks (range 3–39) of rIFN therapy (
Table 1).
The clinical neurological status was normal in all patients before the start of rIFN therapy. Patient #5 had an ischemic cerebral insult after 16 days of treatment, resulting in motor aphasia and a right hemiparesis, accentuated in the arm. Under continued interferon treatment, these symptoms showed an excellent remission tendency and subsided during the following month. None of the other patients manifested overt neurological symptoms. In particular, no signs of polyneuropathy could be detected.
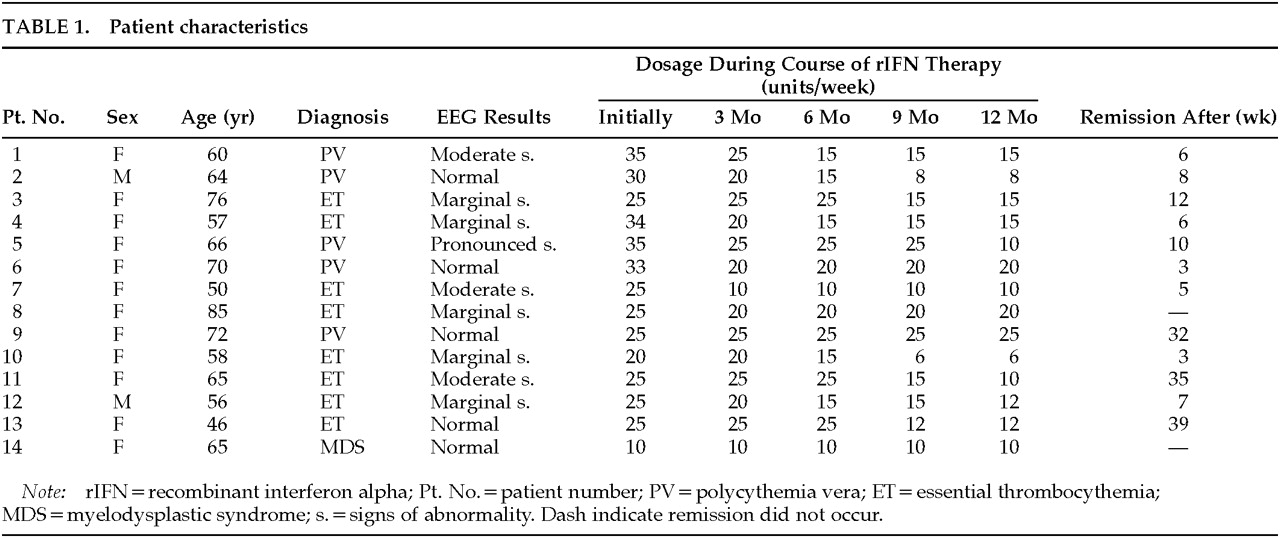
The results of psychometric testing and muscular strength testing are depicted in
Figure 1. The indices of attention (pre-IFN: median 1, range 0–14) and memory (pre-IFN: median 3.5, range 0–8) had improved significantly (
P<0.01 and
P<0.05, respectively) by the third month of treatment. The median speed of spontaneous tapping (pre-IFN: median 69, range 20–120) almost doubled, reaching significance (
P<0.05) after 6 months. Although the initial results of the arithmetic test covered the entire range of possible correct answers (median 2.5, range 0–3), they remained almost identical on retesting. Muscular strength (pre-IFN: median 17 kp (kilopond), range 9–22) increased only slightly, but consistently enough to yield significant results (
P<0.05) after 6 months of rIFN treatment.
Prior to rIFN therapy, EEG was normal in 5 patients but revealed marginal, moderate, and pronounced signs of abnormality in 5, 3, and 1 patients, respectively (
Table 1). After 9 months of treatment, Patient #9 showed moderate diffuse pathological changes without any clinical correlates. None of the other patients' findings changed significantly (data not shown).
All parameters of electroneurography were within the normal range.
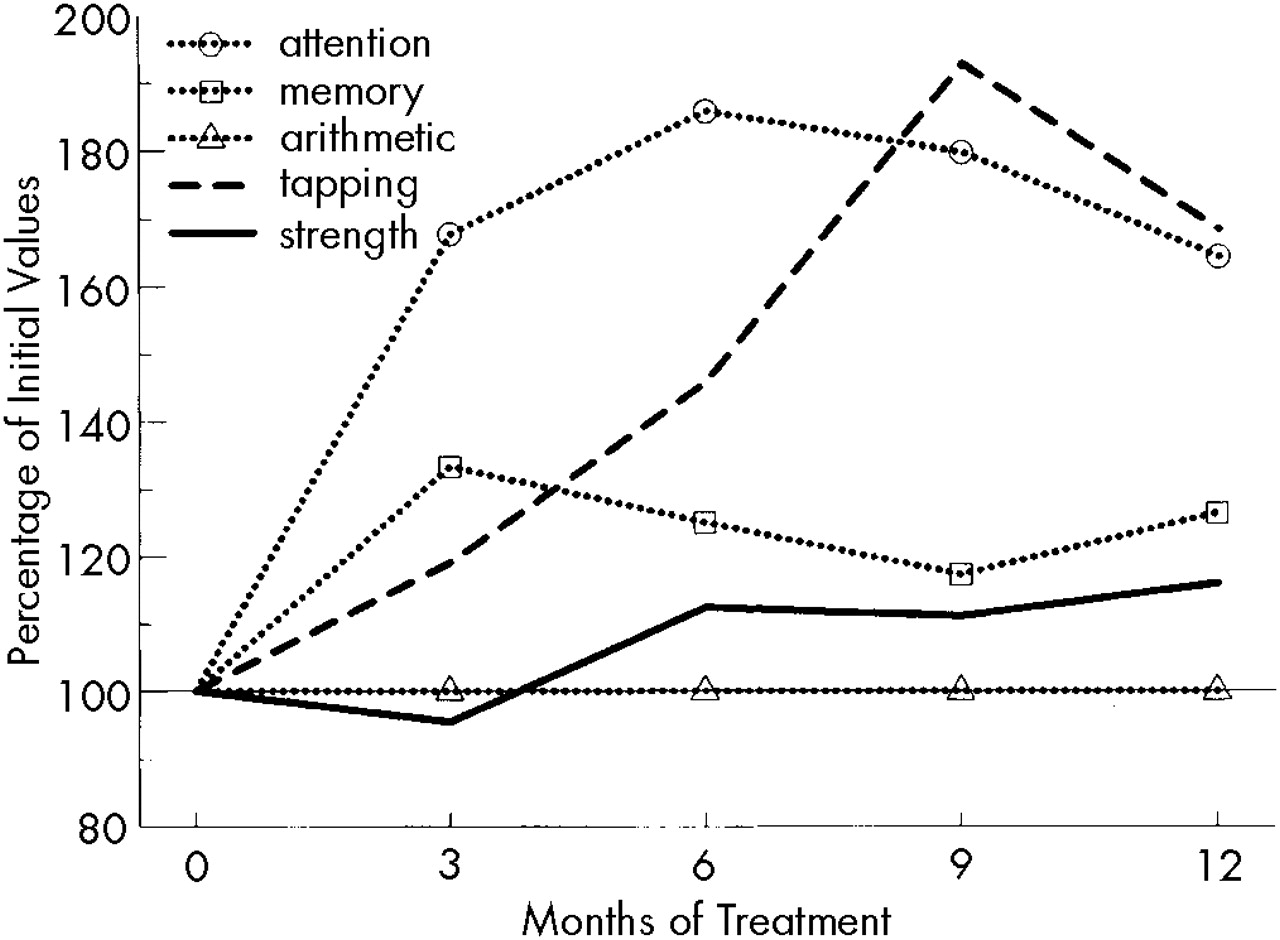
Figure 2 shows the results of VEP. Although the P2 latencies remained constant, P2-N3 amplitudes—after a slight decrease (not significant) during the first 3 months—increased significantly (
P<0.05) after 6 months of rIFN therapy.
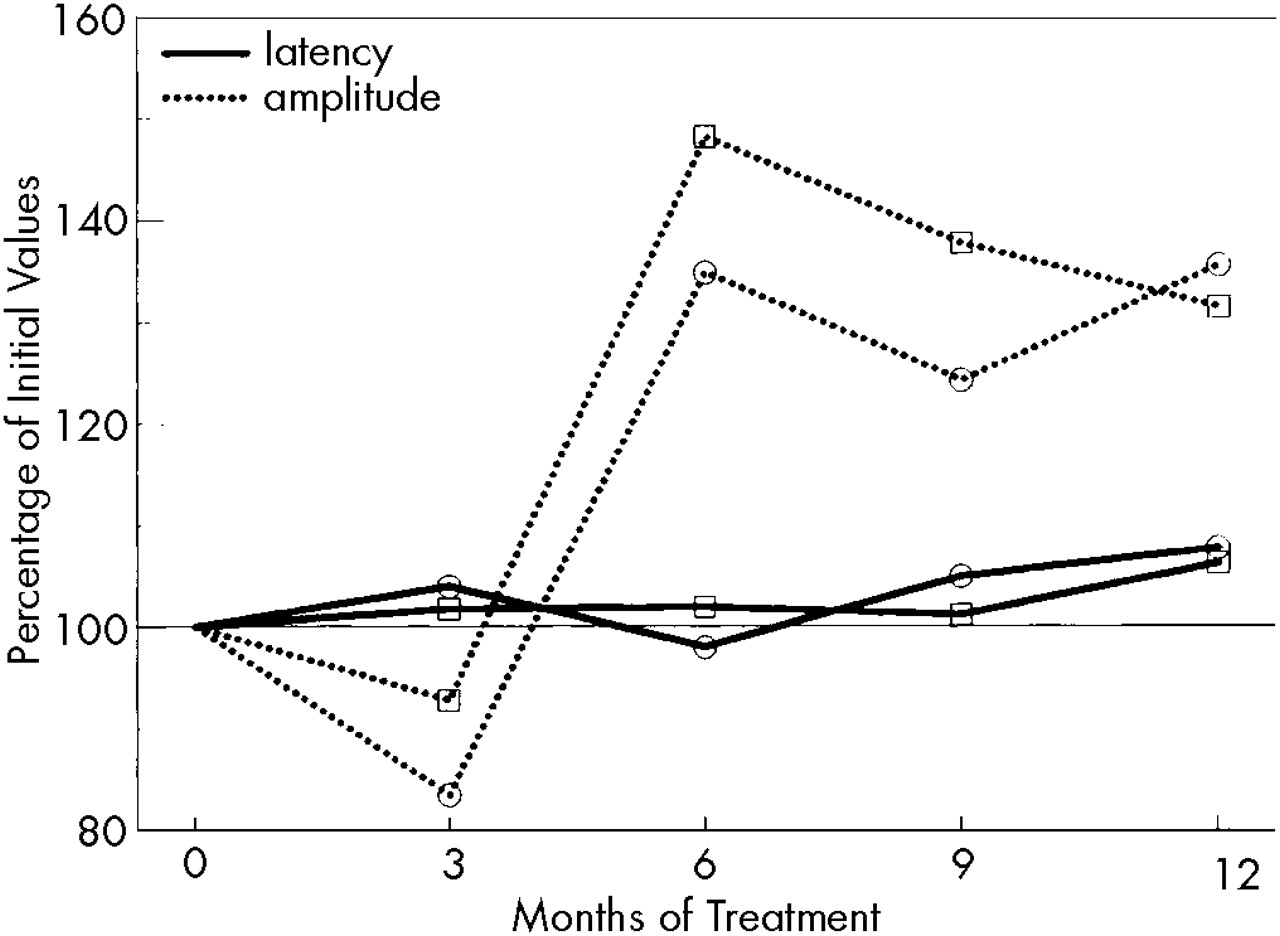
The CMAP-amplitude of the peroneal nerve (pre-IFN: median 6,500 μV, range 4,600–15,000) had increased significantly (
P<0.05) by the sixth month of treatment; the equivalent increase of the median nerve (pre-IFN: median 12,500 μV, range 6,600–15,000) reached the level of significance only after 12 months of rIFN therapy (
P<0.1 for months 6 and 9). Neither motor (pre-IFN: median nerve, median 55.5 m/s, range 51–62; peroneal nerve, median 46 m/s, range 39–54) nor sensory antidromic NCV, nor distal latency (pre-IFN: median nerve, median 3.9 ms, range 3.4–4.8; peroneal nerve, median 4.75 ms, range 3.4–6) changed significantly during the course of treatment (
Figure 3). Regarding the observed significant changes, it must be emphasized that practically all improved values still remained within normal limits.
Evaluation of significant correlations (P<0.05) focused on rIFN dosage and duration of remission, eliminating the intercorrelation between these two parameters. Attention, memory, and tapping correlated with remission duration (r=0.620, r=0.607, and r=0.461, respectively). On the other hand, the CMAP-amplitude (median nerve, r=–0.535; peroneal nerve, r=–0.375, P<0.1) and strength (r=–0.721) inversely correlated with the dose of rIFN. No correlation was found with the parameters of visually evoked potentials.
DISCUSSION
Our results basically confirm the rare incidence of toxic neurological side effects of rIFN that has been reported by several authors.
13,18,19 The only clinically overt neurological manifestation observed in our patient group (one case of a cerebral insult) occurred during an early stage of induction therapy and under the highest dosage applied in this study. After dosage reduction, the patient recovered and eventually achieved remission. However, at the start of rIFN treatment the patient was 66 years old, suffered from polycythemia vera with excessive thrombocytosis, and presented with pronounced pathological signs in his EEG. The treatment association of this neurological affection during rIFN therapy, therefore, remains open to question.
Although 13 of 14 patients showed no neurological deficits during rIFN therapy, even subclinical impairments of brain functions, such as vigilance, could be hazardous to the patient or detrimental to his or her well-being. Two psychometric studies
15,25 revealed slight to moderate deteriorations of various psychological functions, and one group
25 reported changes in emotional life and personality within the first week of treatment. Since our investigational design did not include the earliest stages of rIFN therapy, we cannot rule out similar effects in our patient population. During long-term treatment, however, attention and short-term memory improved, whereas a learned higher mental capacity, arithmetic, was not affected. Attention, memory function, and tapping correlated significantly with the duration of remission. An improvement of these higher brain functions may be due to a gain in the level of arousal as an effect of the rIFN treatment, an effect similar to that observed by Mapou et al.
26Enhanced arousal could also explain the observed increases in the P2-N3 amplitude of VEP. Considering the report of pathological VEP under high-dose rIFN,
21 our observation of a small, statistically insignificant drop in the amplitude at month 3 could indicate some initial detrimental effect of rIFN.
Electroneurography showed that sensory and maximal motor NCVs remained essentially unchanged. These findings are consistent with reports in the literature.
21 CMAP-amplitudes increased significantly, as did muscular strength. The improvement in these electrophysiological parameters might be influenced by several factors, such as decreasing dosages of rIFN during the course of therapy, duration of rIFN exposure, or improvement of platelet counts.
Our patients were afflicted with a disease entity that rendered them prone to disturbances of the microcirculation. Successful treatment, therefore, is likely to have benefited the brain tissue and may even have overcome possible subduing side effects of rIFN. Previous observations of microcirculatory disturbances in the extremities of patients treated for thrombocytosis in myeloproliferative diseases have shown highly significant improvements after achievement of complete remission.
22 The timing of this therapeutic effect was comparable to the currently observed gain in muscle power and increase of CMAP-amplitudes.
Our positive findings of an absence of serious cognitive dysfunctions during long-term rIFN are in line with the results of a large randomized quality-of-life study in myeloma patients.
27 In that study, IFN-treated patients did not observe a reduction of their cognitive and emotional functions in comparison to the control group. The present findings are at variance with the conventional wisdom that IFN commonly causes cognitive dysfunction. Although all observed changes remain at a subclinical level, the tests performed in the present study may be useful tools in monitoring the effects of rIFN therapy in patients with microcirculatory impairments. They also show that in these patients, long-term therapy with low-dose rIFN does not increase the risk of neurological or neuropsychological side effects, but rather diminishes it.