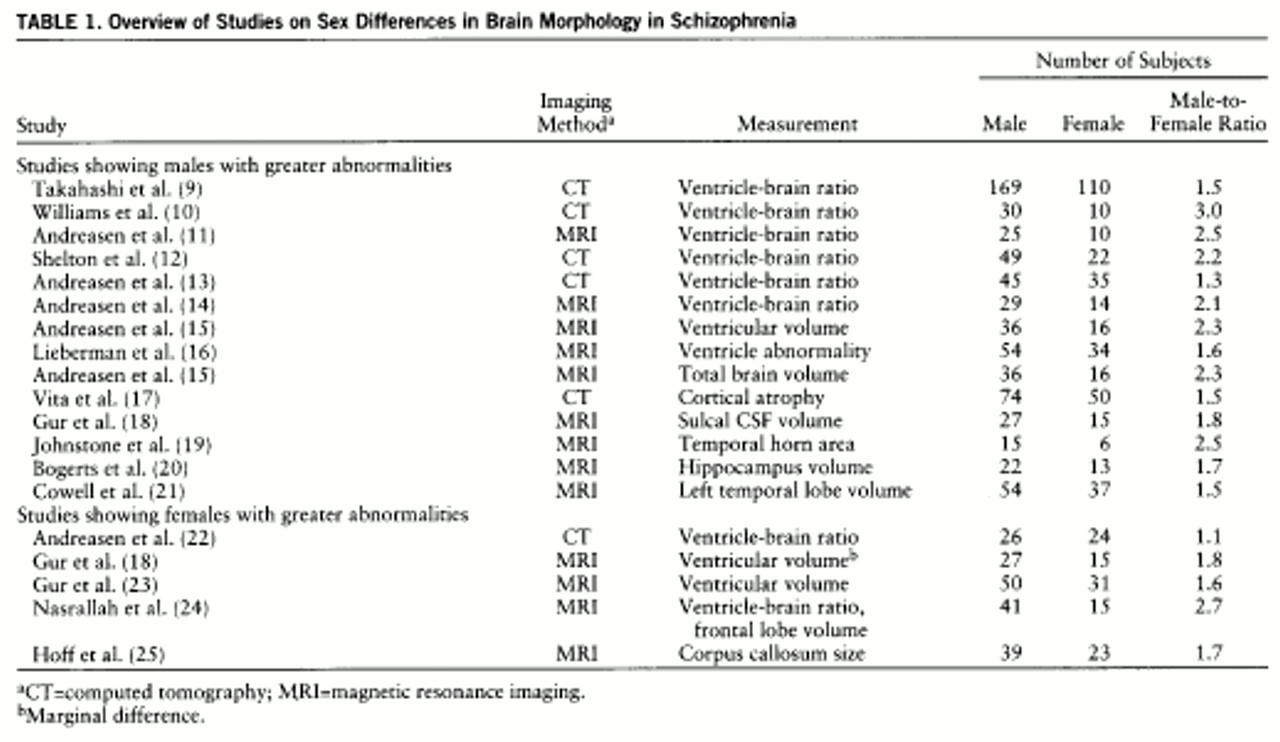
The literature on differences between the sexes in schizophrenia continues to grow. Many of the phenomenologic variables that differentiate male and female patients indicate that males are more likely to manifest more severe forms of the illness. Males have an earlier age at onset (
1,
2), poorer premorbid adjustment (
3), more negative symptoms (
4), more maternal obstetric complications (
5), and poorer response to treatment (
6). Similarly, all of these same variables have also been associated with severity of brain abnormalities, particularly ventricular enlargement (
7,
8). Taken together, these studies support the notion that male patients may have a more severe manifestation of the illness than female patients, which might be reflected in more structural brain abnormalities. However, the literature on sex differences in brain morphology among schizophrenic patients is conflicted.
METHOD
Eighty patients (40 male and 40 female) and 80 comparison subjects matched to them by sex and age were studied. The patients were drawn from consecutive admissions to the inpatient unit of the University of Iowa Mental Health Clinical Research Center. As the admission ratio to the center is approximately four male patients for every one female patient, our male study group was established quickly compared to the time it took to complete our group of 40 female patients. Sixteen of these female patients had been previously used in a study evaluating brain morphology in schizophrenia (
15). The male study group did not overlap with that in any previous study. All patients had a diagnosis of schizophrenia, as defined by DSM-III-R criteria, based on consensus of at least three research psychiatrists (P.N., M.F., N.C.A.).
Sociodemographic and symptom data were obtained from the Comprehensive Assessment of Symptoms and History (
29). Symptoms were rated as the worst in the past month with use of the Scale for the Assessment of Negative Symptoms (SANS) (
30) and the Scale for the Assessment of Positive Symptoms (SAPS) (
31) within the comprehensive assessment instrument. An overall rating of illness severity was calculated by summing all global scores from the SANS/SAPS. To determine total lifetime exposure to neuroleptics, we used a measure called neuroleptic dose-years, which accounts for duration and dose of neuroleptics used. The reliability and validity of this measure have been previously published (
32).
The comparison group consisted of healthy volunteers who were recruited from the community through newspaper advertisements. Potential comparison subjects were evaluated with an abbreviated version of the Comprehensive Assessment of Symptoms and History and were excluded if there was any history of psychiatric illness. They were also excluded if they had a family history of schizophrenia. Patients and potential comparison subjects were excluded if they had a lifetime history of serious head trauma, neurological illness, serious medical or surgical illness, or recent heavy psychoactive drug use or abuse.
After a complete description of the study, all subjects gave written informed consent.
MRI scans were obtained with a T
1-weighted, three-dimensional spoiled-gradient/recalled acquisition sequence on a 1.5-T GE Signa scanner (TE=5 msec, TR=24 msec, flip angle=40°, number of excitations=2, field of view=26 cm, matrix=256×192 pixels) in the coronal plane, yielding 124 contiguous slices that were 1.5 mm thick. Processing of the images after acquisition was done using a locally developed family of software programs called BRAINS (acronym for Brain Research: Analysis of Images, Networks, and Systems) (
33). Details of the image analysis methods have been published elsewhere (
33–
39).
Briefly, a three-dimensional data set was created, and the images were realigned and resampled. At this point, two major variables were created: total brain tissue volume and total CSF volume (broken down into surface or intersulcal CSF and ventricular CSF). Regional measurements were then ascertained by linear transformation into Talairach atlas space in which all of the boxes were assigned to specific brain regions: frontal, temporal, parietal, and occipital lobes. This stereotaxically based method of automated measures has been reported by others to be efficient and accurate for whole brain measures (
40). In addition, our group has previously reported a study in which automated measures of subregions (lobes) were compared with manual tracings (the “gold” standard) in study groups ranging from 20 to 64 men and women, both patients with schizophrenia and healthy subjects. The automated measures were found to be accurate in comparison with the gold standard (
39).
All measures of general and regional brain volumes were subjected to analysis of covariance (ANCOVA) with diagnosis and sex as between-subject factors and height as a covariate. Follow-up t tests for adjusted means were used for the measures that showed significant main effects of diagnosis. In light of our specified hypothesis based on the results from a previous study group (
15), one-tailed tests were used.
RESULTS
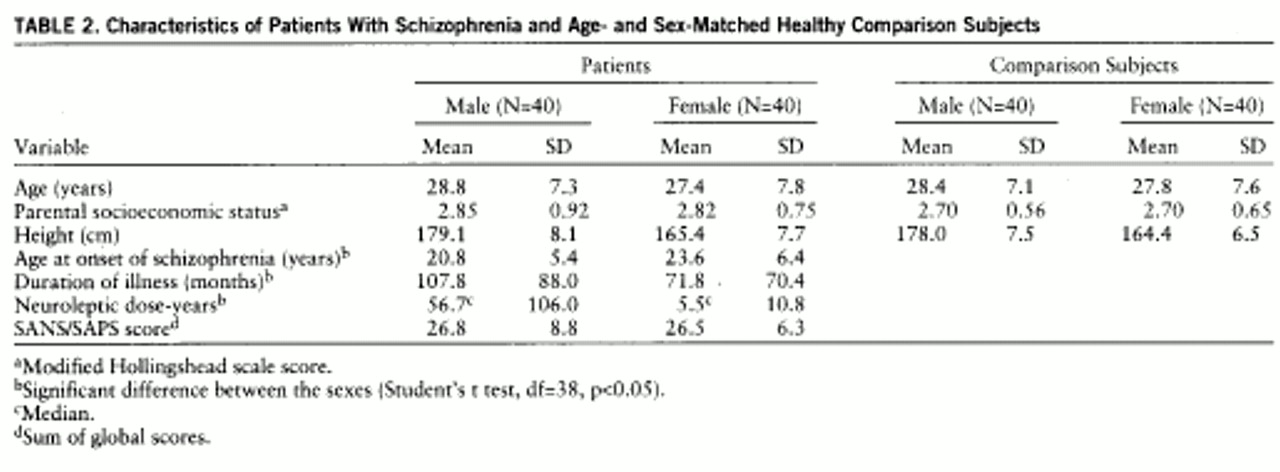
Sociodemographic information on the patients and the comparison subjects is presented in
table 2. As mentioned, the groups were matched by sex and age. There was no significant difference between the two groups in the socioeconomic status of their parents. Also, across the sexes there were no significant differences in age or in the socioeconomic status of parents. Phenomenologic data on the male and female patient groups are also presented in
table 2. There was a significant difference between male and female patients in age at onset and duration of illness. Male patients had an earlier age at onset than female patients, and because the two sexes did not differ in age, the males had a longer duration of illness. In addition, there was a significant difference in medication treatment, since the male patients had a much higher number of neuroleptic dose-years than did the female patients. This variable is reported as the median dose-year because there were large outliers in the means for the male group. However, the male patients and female patients did not differ on the symptom ratings, suggesting that they were similar in the severity of their illness.
MRI variables were analyzed with an ANCOVA on the entire study group. There were significant main diagnostic effects for total tissue volume (F=2.85, df=1,155, p=0.05), frontal lobe volume (F=7.44, df=1,155, p=0.004), temporal lobe volume (F=5.76, df=1,155, p=0.008), and total CSF volume (F=23.01, df=1,155, p=0.0001), including both cortical CSF volume (F=20.64, df=1,155, p=0.0001) and ventricular CSF volume (F=11.60, df=1,155, p=0.0009). All tissue volumes were decreased and CSF volumes were increased in the patients. There were no diagnostic effects for total tissue, parietal lobe, occipital lobe, or cerebellum tissue volumes. There was a significant sex-by-diagnosis interaction for one measure, ventricular volume (F=4.66, df=1,155, p=0.03), indicating the clear presence of a sex difference in this well-matched study group for the most widely studied measure of brain pathology that has been evaluated in schizophrenic subjects.
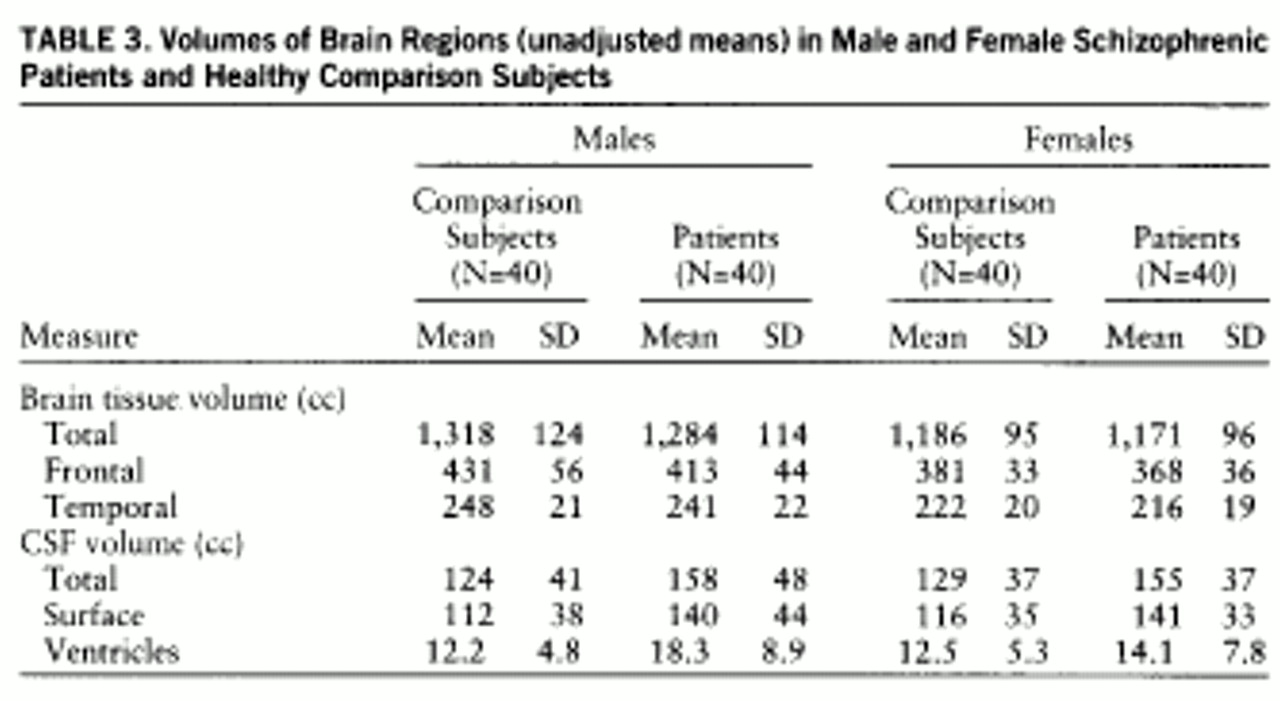
Table 3 presents the breakdown of volumes by sex, showing the unadjusted means for the measures that had a significant diagnostic effect.
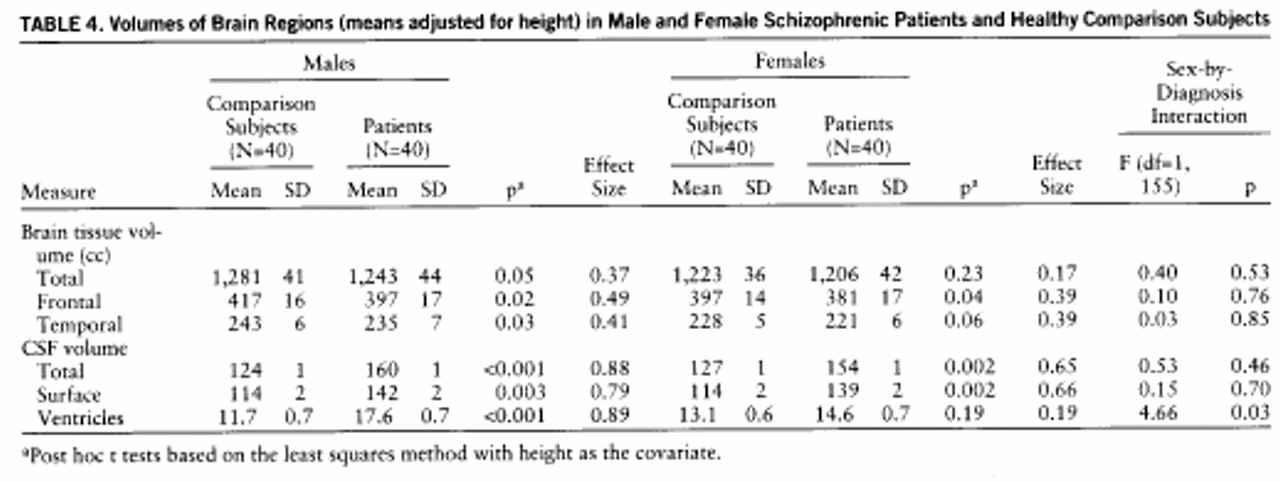
Table 4 presents the same measures but shows the means adjusted for height. As can be seen in comparing the two tables, the covariate had the most robust effect on the tissue volume measures as compared with the CSF measures. The results of the post hoc tests in which the adjusted means were used show that the male patients had significant differences from the male comparison subjects on all measures—total tissue, frontal lobe tissue, and temporal lobe tissue and greater total CSF, including surface and ventricular CSF. In contrast, the female patients had significantly less frontal lobe tissue, greater total CSF and surface CSF, and a trend toward smaller temporal lobes than the female comparison subjects. However, the female patients did not show significant differences in total brain tissue or ventricular volume.
Not only did the female patients show a smaller number of significant abnormalities, but even the structures that were abnormal in comparison with those of the healthy subjects appeared to have less prominent abnormalities than the same structures in the male patients. This can be illustrated by comparing effect sizes, which is a way of showing the size of a difference between two groups based on the average intersubject variability. Effect sizes of 0.20 are considered small; 0.50, moderate; and 0.80 or greater, large (
41). Effect sizes for the male and female groups are presented in
table 4. Although overall the effect sizes for males and females were quite similar, all effect sizes for the female group were smaller than those for the males. For example, although a decrease in frontal lobe volume was significant for both the male and female patient groups, the male group had a larger effect size (0.49) than the female group (0.39). Although the difference between these two effect sizes is not statistically significant (i.e., no significant diagnosis-by-sex interaction), there appears to be a biologically important pattern in which the female patients manifest more subtle brain abnormalities than the male patients.
Because the male patients had a longer duration of illness and more cumulative neuroleptic exposure, the relationship of these two variables and the measure most prominently affected by sex, total ventricular volume, was evaluated. Spearman correlations of duration of illness and dose-years showed no relationship to ventricular volume, partialed for height and age (both having a significant effect on ventricular volume): for duration of illness and ventricular volume, r=–0.07, N=80, p=0.54; for neuroleptic dose-years and ventricular volume, r=–0.01, N=80, p=0.91. Therefore, the phenomenologic differences between the male and female patients did not appear to account for the effect of sex on ventricular enlargement.
DISCUSSION
These findings confirm the presence of significant differences in structural brain abnormalities between male and female patients with schizophrenia. This was most prominent for ventricular enlargement, with male patients showing a strikingly greater volume of CSF compared with healthy males, while female patients compared with healthy female subjects had a very minimally greater ventricular volume. In addition, a pattern emerged in which the abnormalities seen in the female patients were consistently more subtle than those seen in the male patients, even on those measures on which the female patients differed significantly from their comparison subjects. Although the finding that male patients had more significant abnormalities than female patients has been documented several times before, this is the first study to have addressed the confounding factor of study groups unbalanced in sex ratio. The fact that our study group had equal numbers of men and women strongly supports the notion that the “male effect” of greater morphologic abnormality is not an artifact due to lesser power because of a relatively smaller female study group.
The current study group had some important differences in the phenomenologic variables that may have had an impact on the findings. First, our female patients were taken from an inpatient population, which typically represents the more severely ill patients, who are often predominantly male (
42). This suggests that the more typical female patient is more likely to be encountered in an outpatient setting. Corroborating this suggestion, the studies that have found female patients to be more impaired than male patients or that have found no sex effect have been based most often on groups drawn from inpatients, usually from institutions. On the contrary, studies that have found male patients to be significantly more impaired have drawn subjects primarily from outpatient populations or from private treatment facilities (
42). The female patients in our study group were, according to the symptom ratings, cross-sectionally equally as ill as the male patients, suggesting that they may have manifested a more severe form of the illness and thus were not “average” female patients. This would tend to have biased our study group toward a finding of no sex difference. Thus, our data may actually be an underestimate of the sex differences that might be found between male patients and a more representative, less ill female group.
Can the sex effect be accounted for by differences in phenomenologic variables such as length of illness or exposure to medication? One possible solution for answering this question is to control for the phenomenologic variable that differs between the sexes, such as age at onset. However, this becomes circular—phenomenologic differences, such as age at onset, are the sex effect. To control for this variable would indirectly be controlling for sex while trying to investigate sex differences. Nevertheless, it is important to explore the impact, if any, of the phenomenologic differences on brain morphology.
The study group did have the typical sex difference in age at onset, with the males manifesting the illness earlier. Therefore, since our groups were of similar age, the duration of illness and total amount of neuroleptic exposure were correspondingly greater among the male patients. However, these two variables were found not to have any significant relationship to ventricular volume. Several other studies have also found that ventricular enlargement is not significantly correlated with duration of illness or degree of neuroleptic exposure (
24,
43,
44). Thus, the longer duration of illness and greater exposure to neuroleptics of the male patients do not appear to account for the sex effect on brain morphology.
Are the sex differences in the phenomenology and brain morphology of schizophrenia indicative of two separate illnesses or of one illness modulated differently across the sexes? In other words, is the sex effect a matter of degree of severity along a continuum, or is the difference a categorical or dichotomous one? A few investigators have suggested that the sex differences seen in schizophrenia are evidence for two separate illnesses, with the disease in females more likely to represent a variant of an affective disorder rather than the more male-like neurodevelopmental illness of schizophrenia (
4,
45). However, for many reasons, it appears that one illness modulated differently across the sexes may be the more likely explanation.
With regard to brain morphology, the sex effect clearly seems to be one of a difference in severity rather than two separate illnesses. It is important to emphasize that the sex effect on brain morphology, as indicated in this study, is a difference of degree rather than pattern. That is, the brains of females with schizophrenia manifest the same pattern of abnormalities as the one we see in males, but to a lesser degree. If this were two separate illnesses, such similarity in the types and regions of abnormality would not be expected.
There are numerous examples in neuroscience of a single brain process, normal or pathological, that is modulated differently in the two sexes. For instance, the normal processes of development, maturation, and aging in the human brain show some sex effects. In early development, the pace of cerebral development is slower in males than in females (
46,
47). During brain maturational processes from early childhood to adolescence, sex-specific morphologic changes can be found; for example, the volume of the ventricles increases significantly over time in males but not in females (
48). Even later, maturational processes—such as myelination in the hippocampus up to 29 years of age—show sex differences, with females having a significantly greater degree of myelin staining than males during this time period, but an equal degree thereafter (
49). Finally, in the aging process, male brains lose tissue volume (
50) and the size of the ventricles increases (
13) at a faster rate than they do in females.
In addition to normal processes, pathological processes, such as disease pathology or response to environmental insult, show distinct sex differences. For instance, males with low birth weight are more likely than their female equivalents to develop intraventricular and periventricular hemorrhage and to experience long-term adverse neurological deficit because of it (
51,
52). This vulnerability to the adverse effects of insult carries into adulthood, as females tend to have a better prognosis after stroke than males (especially in language skills) (
53). Males also appear to be prone to manifest neurodevelopmental disorders. The majority of the childhood neurodevelopmental syndromes occur more commonly in males than in females; these include mental retardation, learning disorders, communication disorders, autism, attention deficit hyperactivity disorder, and enuresis (DSM-IV). It is important to note that these are not exclusively male disorders; they do occur in females, but less frequently and possibly to a less severe extent. For example, severe mental retardation is also more common in males (
54). These examples indicate that it is a common phenomenon for brain processes, both normal and pathological, to be modulated differently by sex, and they support the idea that a single pathophysiology (schizophrenia) can be manifested in different degrees of incidence and severity of illness depending upon sex.
What is the pathophysiology of the sex effect? In other words, what is it about female brains that tends to make them less vulnerable to pathological processes? In the case of schizophrenia, some writers have pointed to the protective effect of high estrogen levels during adolescence and adulthood as a possible mechanism to account for some of the sex differences in phenomenologic variables (
55). However, as an explanation for the sex effect on brain morphology in schizophrenia, this is probably an oversimplification. Probably much more important is the role that sex hormones play in early development of the brain, leading to sex differences in brain function and brain morphology at both the microscopic and macroscopic levels (
56–
58). These differences are most prominent in areas of the brain, such as the hypothalamus, that control sexual function and behavior. However, some research suggests that this process also leads to sex differences seen in other types of behavior and possibly even cognition (
59). Although there has been much research to support the notion that the human brain differs between the sexes (
59,
60), the degree to which the human brain is truly sexually dimorphic is still unclear (
61). Yet it seems likely that the process is similar to that in animals, in which the influence of sex hormones at a critical point in development initiates a process that leads to sex differences in the structure, function, growth, and maturation processes of the brain. The end result of these sex differences is a female brain that seems more resistant than the male brain to a variety of insults and disease processes such as schizophrenia, leading to a slightly less severe illness—in terms of both phenomenology and brain morphology—in females.
Finally, although the studies of the morphologic brain abnormalities in schizophrenia have been voluminous, there is no doubt that the abnormalities we have seen in anatomy are often subtle, and therefore the literature has inconsistent findings. There are many confounding elements, the least of which may be heterogeneity of the illness. As is suggested by this study, sex may be an important confounding factor in the study of brain morphology in schizophrenia and should be considered as one of the first things to account for when one is trying to study more homogeneous groups of patients.