Sexual dysfunctions, including loss of desire, erectile dysfunction, delayed ejaculation, and anorgasmia, are widely accepted as frequent side effects of treatment with selective serotonin reuptake inhibitor (SSRI) antidepressants (
1). Studies of treatment-emergent sexual dysfunction, however, have ignored the contribution of other factors. High community prevalence rates of inhibited orgasm (4%–10%), erectile dysfunction (4%–10%), inhibited female arousal disorders (11%–48%), and premature ejaculation (35%) have been reported (
2), as has an association between sexual dysfunctions and depression (
3–
6). Despite these potential confounds, many studies of sexual dysfunction have been cross-sectional, have relied on patients' attribution of sexual dysfunction after initiation of treatment, and have excluded patients with baseline dysfunction.
The current study assessed sexual functioning in men and women with chronic depressive disorders before and after a 6-week course of sertraline or paroxetine. We asked two questions: 1) What sexual dysfunctions do chronically depressed men and women experience before and after SSRI treatment? 2) Do changes in depression during treatment influence sexual functioning?
METHOD
Subjects were recruited from adults who contacted the Cornell University Medical Center in response to a newspaper article on dysthymia. Treatment was free, but subjects paid for medication. Upon recruitment, written informed consent was obtained. Inclusion criteria were age greater than 17 years, a score of at least 12 on the Hamilton depression scale (
7), and DSM-III-R diagnosis of chronic major depression, double depression, or dysthymia. Subjects who had undergone surgery, were taking other medications, or had a confounding medical illness, neurological illness, or history of alcohol or drug abuse were excluded. At baseline, trained raters administered the axis I portion of the Structured Clinical Interview for DSM-III-R (
8) and the Hamilton depression scale. Subjects were randomly assigned to 6 weeks of treatment with sertraline or paroxetine and received standard clinical management at 2-week intervals. The Hamilton depression scale was readministered after the 6-week trial by the treating physician. Sexual function was measured by using an early version of the Arizona Sexual Experience Scale, a gender-specific, five-item, self-report measure (
9). Subjects rate, on a 5-point Likert scale, their current level of sexual drive, psychological arousal, physiologic arousal (erection or vaginal lubrication), ease of orgasm, and orgasm satisfaction. Low scores depict sexual response to be “as strong, easy, or satisfying as ever,” while high scores depict the absence of response, e.g., “no sex drive,” “never aroused,” or “never reach orgasm.” Higher scores, therefore, indicate greater sexual dysfunction.
Pretreatment differences between men and women were assessed by using the Mann-Whitney test. Within-group changes in item and total scores on the Arizona Sexual Experience Scale from before to after treatment were analyzed by using the Wilcoxon test. The associations between changes in scores on the Arizona Sexual Experience Scale and the Hamilton depression scale were evaluated by using Spearman correlation coefficients. Internal consistency reliability of the Arizona Sexual Experience Scale was evaluated by using Cronbach's coefficient alpha.
RESULTS
Thirty-one subjects (13 men, 18 women) completed the initial assessment and entered the trial. Two men and one woman did not complete treatment. Three female subjects were excluded because of sexual inactivity. The remaining 25 subjects (11 men, 14 women) are analyzed here.
Most subjects were white (84%, N=21), college graduates (80%, N=20), and involved in a sexual relationship (76%, N=19). The mean ages of the men and women were 49.3 years (SD=9.7) and 40.6 years (SD=10.3), respectively. Sertraline was given to five women (mean dose=60.0 mg/day, SD=13.7) and six men (mean dose=66.7 mg/day, SD=38.8). Paroxetine was given to nine women (mean dose=25.0 mg/day, SD=7.6) and five men (mean dose=20.0 mg/day, SD=0.0). Mean baseline and posttreatment Hamilton depression scale scores, respectively, were 21.1 (SD=6.3) and 7.6 (SD=6.9) for men and 23.6 (SD=5.9) and 9.1 (SD=7.1) for women. No significant gender differences were noted for education, race, partner status, mean drug dose, or Hamilton depression scale scores. Internal consistency reliability of the Arizona Sexual Experience Scale for this cohort as measured by Cronbach's coefficient alpha was 0.88. After 6 weeks of treatment, 64% (N=9) of the women and 73% (N=8) of the men met response criteria (Hamilton depression scale score of less than 10 and more than a 50% improvement in score from baseline).
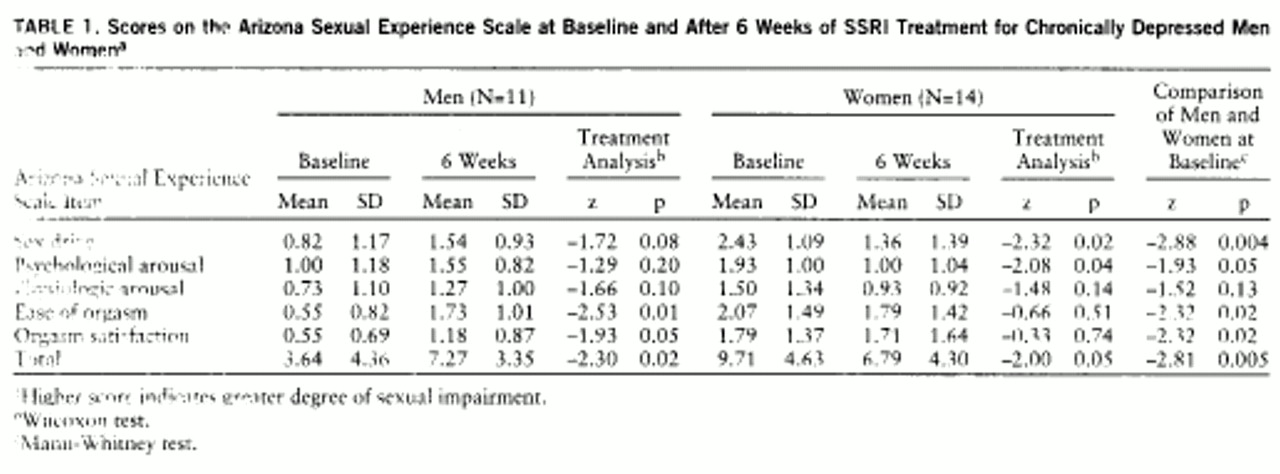
Mean item and total scale scores of the Arizona Sexual Experience Scale at baseline and after 6 weeks of treatment, stratified by gender, are reported in
table 1. The baseline scores on the Arizona Sexual Experience Scale indicated sizable and significantly greater impairment in women for all items except physiologic arousal; therefore, subsequent analyses were conducted separately by gender. After 6 weeks of SSRI treatment, sex drive and psychological arousal improved significantly in women, although they reported no change in vaginal lubrication (physiologic arousal), ease of orgasm, or orgasm satisfaction. In men, ease of orgasm and orgasm satisfaction significantly worsened, and there was a trend toward worsening sex drive. Men reported no change in psychological arousal or physiologic arousal (erections). Global sexual functioning, as measured by total score on the Arizona Sexual Experience Scale, improved significantly in women and worsened significantly in men. Differential medication effects upon sexual functioning were not analyzed because of group size constraints.
Correlations were computed to determine the strength of the linear relationship between scores on the Hamilton depression scale and the Arizona Sexual Experience Scale. We hypothesized that changes in scores on the Arizona Sexual Experience Scale would be strongly correlated with changes in scores on the Hamilton depression scale in women but not in men. Significant improvements in sexual functioning in women would, therefore, correlate with improvements in depression, while significant deterioration in sexual functioning in men would not. For the 14 women, correlations between changes in scores on the Arizona Sexual Experience Scale and the Hamilton depression scale approached significance for psychological arousal (rs=0.51, p=0.06) but not for desire (rs=0.15, p=0.61) or global sexual functioning (rs=0.38, p=0.24). For the 11 men, correlations between changes in scores on the two scales for orgasm (rs=–0.25, p=0.49), orgasm satisfaction (rs=–0.51, p=0.14), and global sexual functioning (rs=–0.29, p=0.42) did not approach significance. A study group size of 30 for each gender would be required to reject the null hypothesis of no correlation (two-tailed alpha=0.05) if a population correlation of 0.50 exists, which thus raises the possibility of a type II error.
DISCUSSION
We believe that this is the first longitudinal study of sexual functioning in chronically depressed men and women that has explored SSRI and depressive illness effects. Depressed women experienced greater sexual dysfunction at baseline and improvement in sexual functioning with treatment, whereas men experienced worsening sexual functioning. Both difficulty reaching orgasm and loss of orgasm satisfaction in men treated with SSRIs appear to be side effects of medication and have been widely reported. The absence of worsening sexual functioning in depressed women treated with SSRIs was unexpected but is supported by clinical experience and data from the product manufacturers of sertraline and paroxetine (1996 prescribing information from Pfizer and SmithKline Beecham, respectively), which have indicated less frequent negative sexual effects in women. Although possibly attributable to a ceiling effect, slower onset, or reporting differences, sexual side effects from SSRI treatment may be less common in women or are overshadowed by the salutary effects on sexual functioning that are associated with treating depression. Our finding of no association between improvement in depression and improvement in sexual functioning after SSRI treatment in women needs to be reassessed in a sample of adequate size.
Our study highlights the need for ongoing research on the impact of depression and pharmacotherapy on sexual functioning. Although limited by brief duration, small study group size, and the absence of a placebo condition and a well-validated instrument, this study suggests that SSRI treatment is of benefit for sexual functioning in chronically depressed women and supports previous findings of orgasmic impairment in men early in treatment. If replicated, this finding would suggest the need for differential treatment strategies. Future studies of sexual dysfunction in depressed subjects should continue to clarify which aspects of sexual functioning are affected by pharmacologic treatments, including differential pharmacologic effects and the effect of depression on sexual functioning.