Childhood-onset schizophrenia is a rare disease affecting children before puberty; i.e., the first psychotic symptoms appear by age 12 years
(1,
2). The pathophysiology of childhood-onset schizophrenia has been investigated in only a few studies, and in general the results support the hypothesis that childhood-onset schizophrenia is in continuity with the adult-onset form of schizophrenia. Clinical studies have shown that the symptoms in childhood-onset schizophrenia are generally similar to and more severe than those seen in adult patients
(2). Other studies have suggested similar patterns of autonomic activity and reactivity
(3) and smooth-pursuit eye movements
(4). Neuropsychological studies
(5,
6) have for the most part shown similar deficits in cognitive processing capacity. Moreover, recent studies
(7,
8) with magnetic resonance imaging (MRI) have shown abnormalities of the lateral ventricles, the basal ganglia, and the mesial temporal lobe, structures that have been classically involved in adult-onset schizophrenia. All these studies suggest that childhood- and adult-onset schizophrenia may share some pathophysiological features and that the earlier onset in childhood-onset schizophrenia is another indication of the fact that this disorder may be a more severe form of the same disease.
Proton magnetic resonance spectroscopy (
1H-MRS) allows in vivo measurement of some aspects of brain biochemistry.
1H-MRS detects signals arising from
N-acetyl-containing moieties, mainly
N-acetylaspartate (NAA). NAA is located within neurons
(9), it increases during development
(10), and it has been shown to be an intraneuronal marker sensitive to a number of pathological processes affecting the integrity of neurons
(11-
14). At relatively long echo time,
1H-MRS also detects signals arising from choline-containing compounds (CHO)
(15,
16) and creatine plus phosphocreatine (CRE). Several previous single-voxel
1H-MRS studies of patients with schizophrenia have shown low NAA signal intensity or concentration in the mesial temporal lobes
(17,
18), in the region of the hippocampus
(19,
20), and in the frontal lobe
(21), but not without controversy
(21-
24).
1H-MRS has evolved to
1H-MRS imaging (
1H-MRSI), a mapping approach that permits acquisition of signals arising from a large number of small single-volume elements
(25). Using this technique, we and others have previously shown low levels of NAA measures in the hippocampal area and the dorsolateral prefrontal cortex of patients with adult-onset schizophrenia
(26-
29). These findings were independent of drug treatment and were consistent with developmental neuronal pathology
(28,
30).
The purpose of the present study of patients with childhood-onset schizophrenia was to investigate whether the hippocampal area and dorsolateral prefrontal cortex show the same neurochemical pattern seen in adult patients with schizophrenia and, if so, the relative extent of the abnormalities.
RESULTS
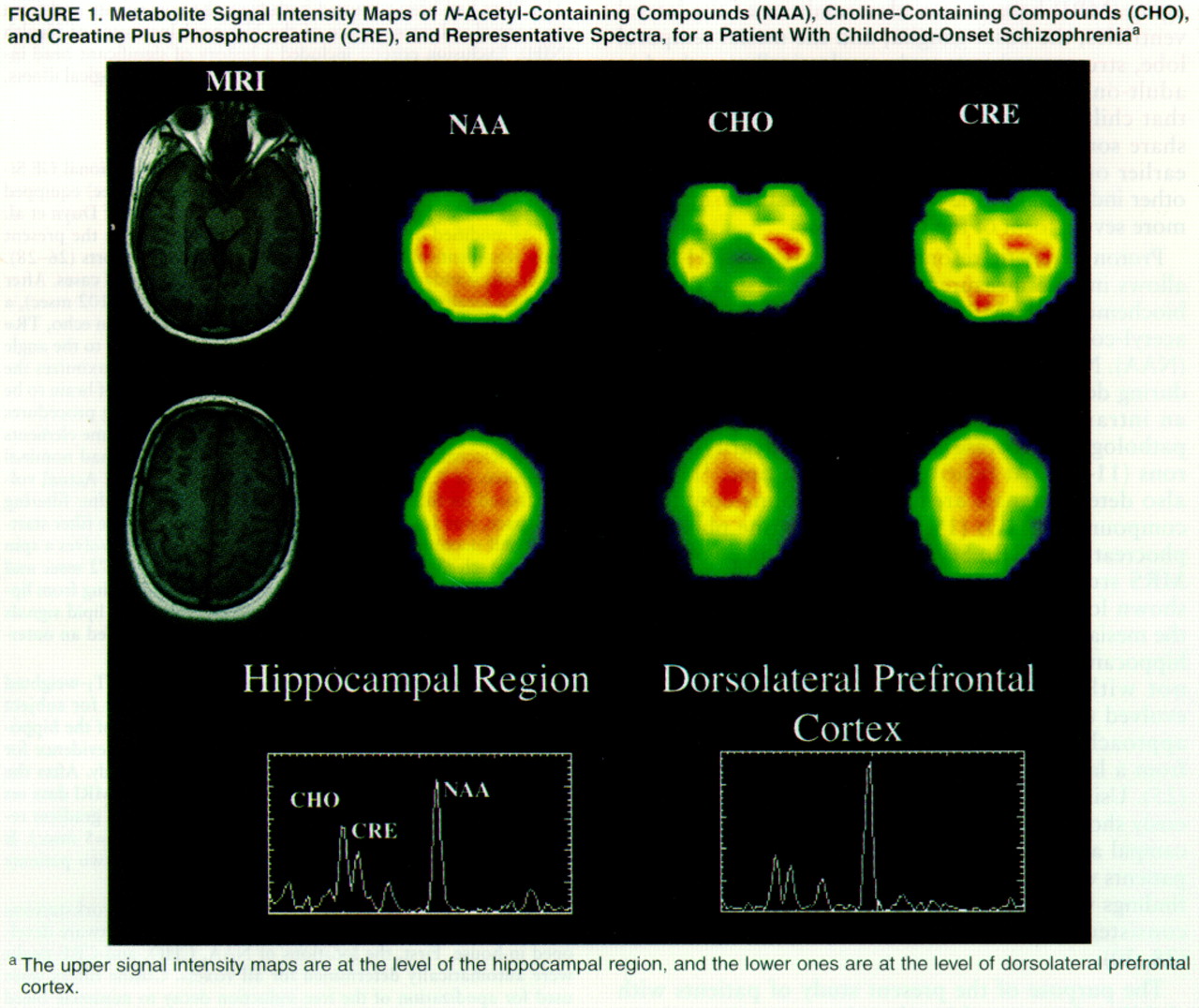
Figure 1 shows metabolite maps of NAA, CHO, and CRE signal intensities with the coaxial MRIs for a patient and representative spectra from the hippocampal area and the dorsolateral prefrontal cortex. The color images are scaled to the highest value of each metabolite signal intensity for each
1H-MRSI slice, so that the pattern of regional distribution of metabolite signal intensities within the same slice can be compared between subjects, although color intensity from the same anatomical location cannot be compared between subjects.
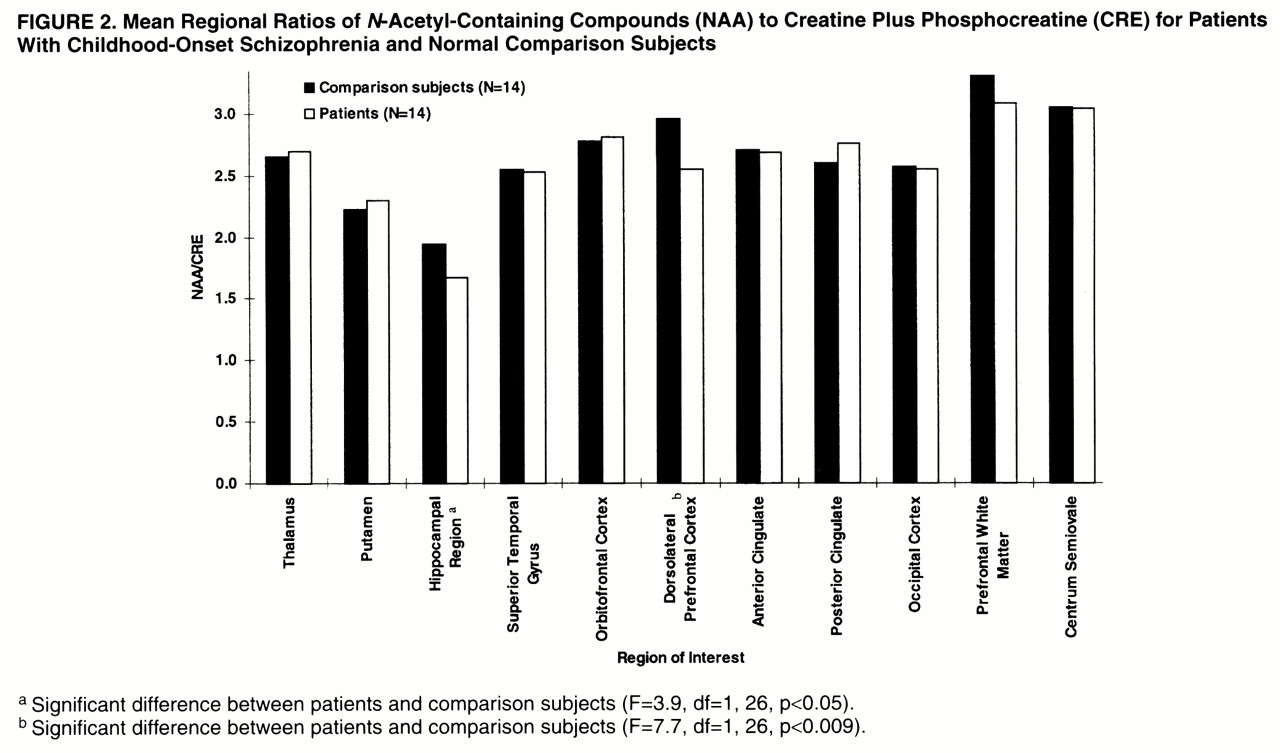
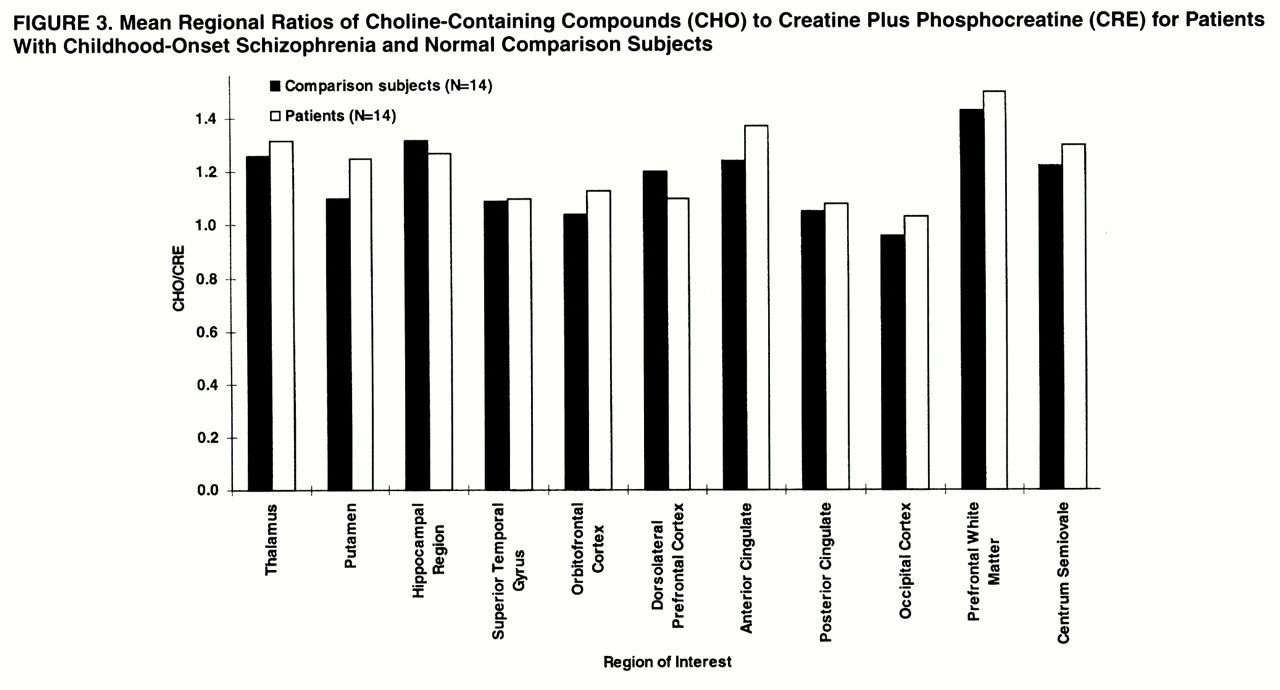
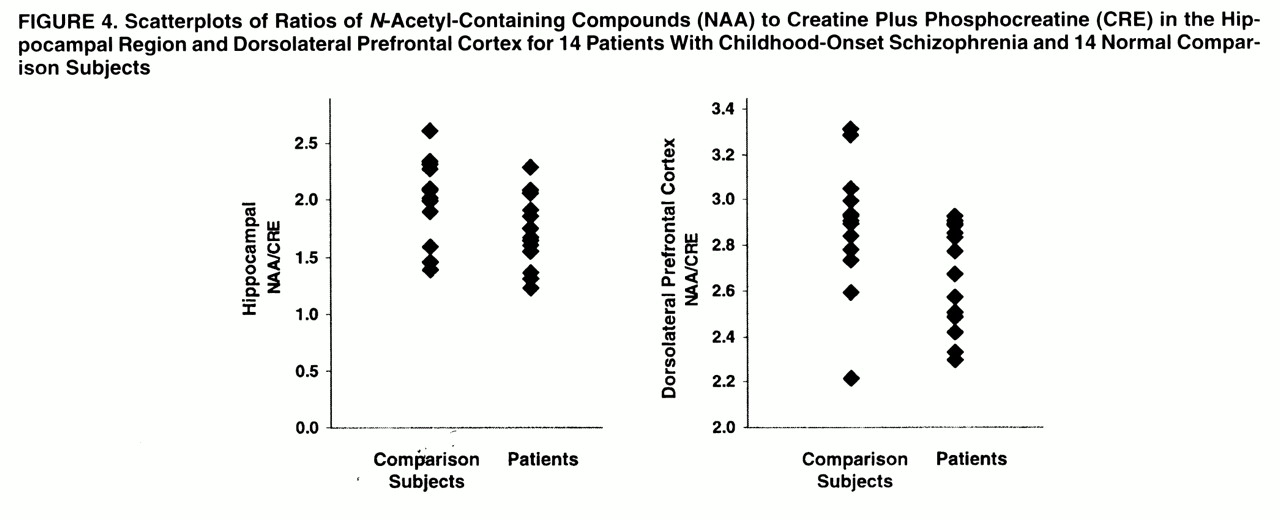
In the hippocampal area (
figures 2,
3, and
4), ANOVAs showed a significant effect of diagnosis for NAA/CRE (F=3.9, df=1, 26, p<0.05) and no effect for NAA/CHO (F=1.3, df=1, 26, p>0.10) and CHO/CRE (F=0.23, df=1, 26, p>0.50). Post hoc analysis showed that the patients had significantly lower bilateral NAA/CRE ratios (p<0.05). No main effect of hemisphere or interaction of diagnosis and hemisphere was found for any metabolite ratio.
In the dorsolateral prefrontal cortex (
figures 2–
4), ANOVAs showed a significant effect of diagnosis for NAA/CRE (F=7.7, df=1, 26, p<0.009), an effect for NAA/CHO (F=3.6, df=1, 26, p<0.06), and no effect for CHO/CRE (F=0.7, df=1, 26, p>0.40). Post hoc analysis showed that the patients had significantly lower bilateral NAA/CRE ratios (p<0.04) and lower bilateral NAA/CHO ratios (p<0.06). No main effect of hemisphere or interaction of diagnosis and hemisphere was found for any metabolite ratio. No main effect or interaction was found in any other region of interest for any metabolite ratio. ANOVA revealed sporadic effects of hemisphere and no interaction of diagnosis and hemisphere. None of the sporadic results, which were not based on a priori hypotheses or previous results, survived an appropriate Bonferroni correction for the number of regions, even at the trend level.
The effect sizes of the NAA/CRE differences were 0.6 for the hippocampal area and 1.3 for the dorsolateral prefrontal cortex. These are comparable to what we previously found
(26) in patients with adult-onset schizophrenia (0.5 and 1.3, respectively).
To evaluate whether the lower ratios seen in the patients are due to differences in the numerator or denominator terms, the mean integrated areas of NAA, CHO, and CRE resonances were normalized to the corresponding mean integrated areas in the centrum semiovale (i.e., hippocampal NAA divided by centrum semiovale NAA, hippocampal CHO divided by centrum semiovale CHO, and so forth). We used the centrum semiovale as a reference because in a previous study
(27) its metabolite ratios showed a low coefficient of variation among several other regions of interest and because it is probably not involved in the primary pathophysiological process of schizophrenia. While low normalized NAA ratios were found bilaterally in the hippocampal area (ANOVA: F=3.5, df=1, 26, p<0.06) and in the dorsolateral prefrontal cortex (ANOVA: F=2.8, df=1, 26, p<0.10), no differences were found for CHO or CRE, further suggesting that the ratio differences between the patients and normal subjects are due to differences in NAA.
No significant correlation was found between the volume of the hippocampal area and the prefrontal lobe and the ratio measures in the hippocampal area and dorsolateral prefrontal cortex (all r values were between –0.14 and 0.32). Spearman tests did not show any significant correlation between NAA ratios in the hippocampal area and dorsolateral prefrontal cortex and the different items of the various rating scales used. A few non-hypothesis-driven correlations emerged, but they were not significant after Bonferroni correction for the number of comparisons. No correlation was found between any clinical variable (age, age at onset of the disease, current neuroleptic dose, years of treatment with neuroleptic drugs, and length of illness) and MRSI measures in the hippocampal area and the dorsolateral prefrontal cortex (all r values were between –0.33 and 0.40).
DISCUSSION
The present study shows that patients with childhood-onset schizophrenia show lower NAA measures in the hippocampal area and the dorsolateral prefrontal cortex suggestive of neuronal involvement exclusively in these two areas. No correlation was found between NAA measures in the hippocampal area and dorsolateral prefrontal cortex and the volume of these structures as measured with MRI, suggesting that they are not related to volumetric losses; this finding is also similar to what we have observed in patients with adult onsets. Moreover, length of illness did not predict NAA levels, suggesting that the NAA differences are not related to progressive pathology. These results are consistent both in the extent and the location of the damage with earlier findings from patients with adult-onset schizophrenia that showed low NAA values in mesial temporal-limbic and prefrontal cortices
(17–
(20),
(26-
29), although some controversy about these findings persists
(21-
24). Overall, the present study adds more emphasis to the thesis of a common pathophysiological process specifically affecting mesial temporal-limbic and prefrontal circuitries in patients with childhood-onset and adult-onset schizophrenia.
The involvement of the hippocampal area and the dorsolateral prefrontal cortex in the pathophysiology of schizophrenia has long been posited
(39). Postmortem studies
(40-
44) have shown anatomical abnormalities consistent with developmental pathology, particularly in the hippocampal region and the prefrontal cortex. Structural studies with MRI have produced results consistent with those from postmortem analyses, showing most often bilaterally lower volumes of the hippocampal region and of the frontal lobe
(45-
50), although negative reports have also appeared
(51). Functional neuroimaging studies have also implicated the dorsolateral prefrontal cortex and its connections with the hippocampal area in the failure of patients with schizophrenia to produce as robust an activation during working memory tasks as seen in healthy subjects
(52). Moreover, previous postmortem,
1H-MRS, and
1H-MRSI studies have shown low NAA values in the mesial temporal lobes
(17,
18), the hippocampus/amygdala area
(19,
26–
28,
53, and the prefrontal cortex
(21,
26–
29,
53) in patients with schizophrenia. While no postmortem study of childhood-onset schizophrenia is available yet, structural imaging studies have shown cerebral abnormalities that are consistent with those seen in adult-onset schizophrenia. The few MRI studies that have assessed the morphology of brain regions specifically in patients with childhood-onset schizophrenia have shown among other findings a lack of the normal right-greater-than-left asymmetry of the hippocampus and a nonsignificantly lower volume of the prefrontal lobe
(7). A study with [
18F]fluorodeoxyglucose and positron emission tomography
(54) showed lower glucose metabolism in anterior frontal regions of patients with childhood-onset schizophrenia than in normal subjects. In another preliminary
1H-MRS study of children with schizophrenia
(55), NAA in the frontal gray matter was significantly lower than normal.
Although our results are consistent with those from previous imaging research in childhood-onset schizophrenia in revealing the anatomy of the circuitry involved in this disorder, some inconsistencies emerge. A recent longitudinal MRI investigation
(8) showed disproportionate progressive changes over 2 years in children with childhood-onset schizophrenia, even an average of 4 years after illness onset. This series of MRI studies has shown progressive reduction in the volumes of temporal and mesial temporal structures (including the hippocampus), of the midsagittal thalamic area, and, indeed, of the whole brain, together with a progressive increase in the size of the ventricles. The large changes seen in these structures of patients with childhood-onset schizophrenia prompted the interpretation that these subjects were in a developmental period particularly sensitive to pathological effects
(8). It was also suggested that this period of development may have been unique in revealing ventricular enlargement that would afterward remain stable
(8). This speculation notwithstanding, the present data, obtained from a similarly aged group that contained patients from those earlier structural studies, show that NAA abnormalities are of similar magnitude to those seen in adult-onset schizophrenia and support the thesis of static pathology affecting neuronal integrity of the hippocampal area and the dorsolateral prefrontal cortex. It should be noted that the lack of a correlation between NAA measures in the hippocampal area and dorsolateral prefrontal cortex and their respective volumes may be an artifact of our relatively small number of subjects, but this negative result was also found in our earlier studies of other study groups
(26,
27). Moreover, we also did not find any correlation between NAA measures and length of illness, which would be expected if the pathology were progressive. In this respect it is interesting that, as in our previous studies of patients with adult-onset schizophrenia
(26-
28), we also did not find any abnormality related to CHO signals. CHO signals can be influenced by several pathological, pharmacological
(56), and physiological processes, including diet
(57). Aware of the limitations associated with the interpretation of the CHO signal, we have previously noted that low NAA values and high CHO values (attributed to gliosis) can be found in neurodegenerative diseases
(58,
59). The current finding of low NAA and normal CHO may imply the presence of neuronal damage unaccompanied by gliosis, such as might occur with a neurodevelopmental defect. Neurodevelopmental pathology would also be consistent with the fact that patients with childhood-onset schizophrenia do not seem to have more profound neuronal damage than patients with adult-onset schizophrenia (as shown by the similar effect sizes). Therefore, it is conceivable that the abnormalities identified in the structural studies (especially those of the hippocampal area) are unrelated to the neuronal compartment associated with NAA signals (i.e., neurons and their processes). These data, however, do not provide further insight into the earlier onset of disease in childhood-onset schizophrenia.
No correlation was found in this study between NAA measures in the hippocampal area and dorsolateral prefrontal cortex and the volume of the hippocampal area and prefrontal lobe. The lack of correlation may again reflect the relatively small number of subjects and the partial volume effects inherent in current spectroscopy techniques, i.e., the spectroscopic signal was measured from an area larger than the real volume. Thus, our MRSI of the hippocampal region and dorsolateral prefrontal cortex might have misrepresented the true anatomical signal. Alternatively, the lack of a correlation between volume and metabolites in our data might be consistent with emerging evidence suggesting that NAA reflects more than neuronal density
(11–
14,
60-
64) and is sensitive to neuronal function.
As in earlier studies, we did not find correlations between NAA measures and treatment with neuroleptics. Although lack of a correlation does not exclude the possibility of some effect of neuroleptics on NAA, it is unlikely that the findings are solely explained by treatment with neuroleptics. In fact, we and others have shown that low NAA measures are found also in patients who have received only minimal exposure to neuroleptics
(18,
28). Moreover, Okumura et al.
(65) performed a study in rats and reported no effect of chlorpromazine on NAA levels. As we reported previously
(26), we cannot exclude that the low NAA values are due to T
1 or T
2 relaxation effects. However, as argued before, such a regionally specific abnormality of NAA is unlikely to arise from such effects. Another limitation of this study is that we did not measure absolute concentrations of the metabolites, thus making the statements about single metabolites not definitive. However, as argued in our previous paper
(26), the pattern of ratio differences (low ratio of NAA to CRE, CHO, and NAA in the centrum semiovale together with no difference in the ratio of CHO to CRE or to centrum semiovale CHO or in the ratio of CRE to centrum semiovale CRE) strongly suggests that the ratio abnormalities are due to NAA.
In conclusion, patients with childhood-onset schizophrenia show low NAA measures in the hippocampal area and the dorsolateral prefrontal cortex, suggesting impairment of neuronal integrity in these areas. These results imply a common pattern of cortical pathology in patients with childhood-onset schizophrenia and patients with adult-onset schizophrenia, further indicating a common physiopathology in these disorders.