Convergent evidence from behavioral, physiological, and neuroanatomical approaches suggests that effects of opioid drugs are mediated by different receptor subtypes, a concept initially proposed by Martin (
1,
2). Among the diversity of opioid receptors described, mu and kappa receptors seem to be of particular relevance for human behavior. Morphine acts upon mu receptors as a typical agonist and produces effects such as analgesia, euphoria, respiratory depression, and miosis. Ketocyclazocine acts upon kappa receptors as an agonist, producing spinal analgesia, sedation, and miosis (
3). The localization of opioid receptors in specific brain areas led logically to the discovery of a series of endogenous substances—the endorphins and the enkephalins—that appear to be responsible for the regulation of pain perception. More recently, these endogenous substances have been hypothesized to be important in the regulation of normal mood states and the mediation of the action of abused substances other than the opioids, such as cocaine (
4–
6).
Most of these observations of opioid effects were conducted with the use of animal models; the differential behavioral effects of opioids in humans have been less extensively studied. Research on humans in which a drug discrimination model was used has demonstrated that the opioid mixed agonist/antagonist butorphanol produces kappa-like effects, while hydromorphone produces primarily mu-like effects (
7). Consistent with this differential discrimination of hydromorphone and butorphanol, these drugs also produce distinct and different patterns of subjective effects (
8).
The implication of these differential effects is that corresponding changes in CNS function should also be distinguishable. We assessed behavioral and physiological effects of butorphanol and hydromorphone and tested the hypothesis that these opioids would produce both different behavioral profiles and different patterns of regional cerebral blood flow (CBF) in comparison with placebo, as assessed by single photon emission computed tomography (SPECT).
METHOD
Nine nondependent opioid-abusing volunteers participated. The inclusion criteria were a more than 2-year history of opioid abuse, absence of dependence symptoms, and absence of any concomitant somatic or psychiatric disorders, as assessed by internist examination and psychiatric interview. Furthermore, absence of any structural abnormalities of the brain, as assessed by subsequent high-resolution magnetic resonance imaging, was required. Written informed consent was obtained, and the protocol was approved by the appropriate institutional review boards for human research.
In phase 1 of the study, behavioral and physiological effects were assessed. The subjects were admitted to the Residential Research Facility at the Behavioral Pharmacology Research Unit of the Johns Hopkins University Bayview Medical Center and underwent six experimental sessions. Following one training session (with saline placebo), five drug conditions were tested: placebo; hydromorphone, 2 and 4 mg/70 kg; and butorphanol, 3 and 6 mg/70 kg. The drugs were injected intramuscularly in random order and in double-blind fashion. The psychophysiological data recorded included respiration rate, skin temperature, heart rate, blood pressure, and pupil diameter. Subjective responses were assessed with adjective checklists and global analog ratings of the magnitude and quality of the drug effects. Clinicians made ratings on the Addiction Research Center Inventory LSD scale, a measure of dysphoric effects. These measures have been shown previously to be sensitive and reliable indicators of acute opioid effects (
8–
10).
In phase 2, effects on regional CBF were assessed. After phase 1, the subjects were transferred under supervision to the Inpatient Clinical Research Center of the Johns Hopkins Hospital, where they remained for 5 days. On days 1, 3, and 5, they received, in a double-blind, random-order design, intramuscular injections of 4 mg/70 kg of hydromorphone, 6 mg/70 kg of butorphanol, or saline placebo. Studies were performed every other day to minimize effects of the radioactive tracer from the previous study remaining bound to brain tissue (
11). Fifty minutes after the injection of the study drug, the subjects were blindfolded and their ears were covered with a silencer to minimize auditory and visual stimulation.
At baseline and 15, 30, and 45 minutes after injection of the study drug (or placebo), subjective effects of the drugs were assessed with the same instruments as in phase 1. Sixty minutes after study drug injection, approximately 20 mCi of the SPECT blood flow tracer [
99mTc]HMPAO (hexamethylpropyleneamine oxime) was injected intravenously. SPECT scans were performed with a triple-head (TRIONIX TRIAD) gamma camera 30–60 minutes after [
99mTc]~HMPAO administration. Each SPECT scan was individually examined for possible artifacts and overall image quality. Image data were analyzed by means of the statistical parametric mapping program SPM 94 (
12–
14). Significant differences in regional CBF between the placebo and hydromorphone conditions and between the placebo and butorphanol conditions were calculated by averaging subjects' images for each condition after correction for the injected dose of radioactivity. The exact location of the voxels with the most significant differences was found with use of the brain atlas of Talairach and Tournoux (
15).
The significance levels cited for the above-mentioned statistical parametric mapping analysis are corrected for the problem of multiple dependent comparisons (as with the Bonferroni method) by a statistical technique based on Gaussian random field theory (
16). Roughly speaking, the statistical parametric mapping test statistic is the peak height of a contiguous set of voxels exceeding a given threshold (
17,
18). Intuitively, this may be understood as a sophisticated and more powerful alternative to a naive Bonferroni correction.
RESULTS
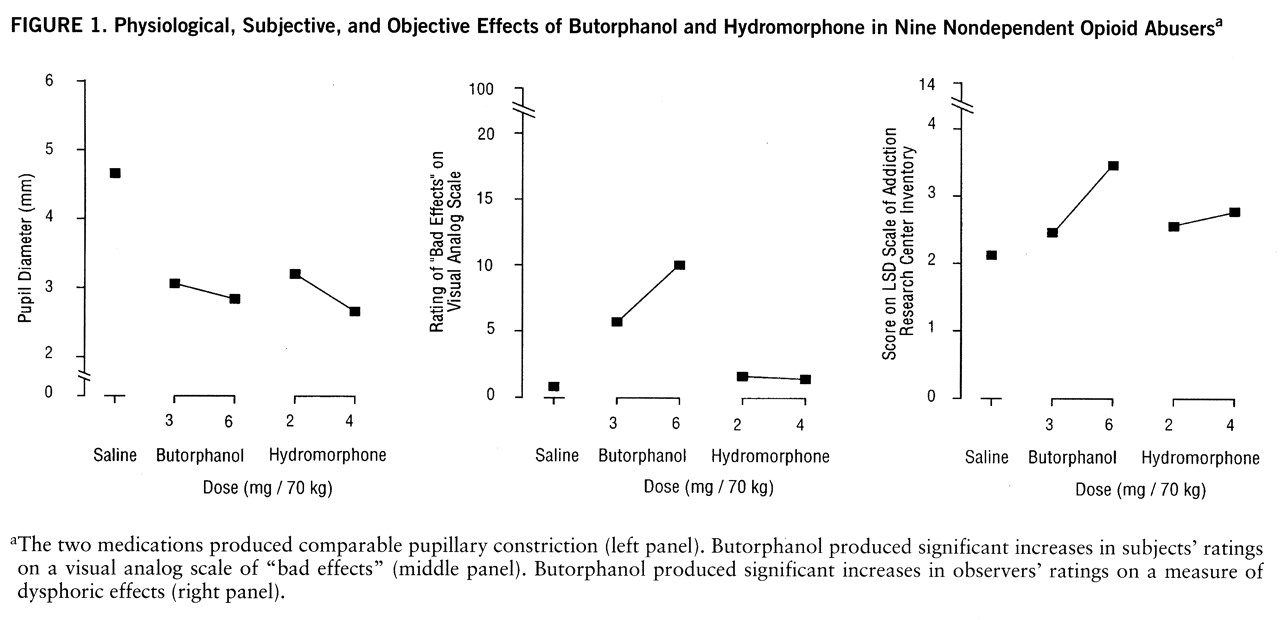
Phase 1: subjective effects. The subjects reported different patterns of response in the hydromorphone and butorphanol conditions. The results for three measures are shown in
figure 1. The two medications produced comparable pupillary constriction. However, butorphanol produced significant increases in subjects' responses on visual analog scale ratings of “bad effects” at both the 3-mg dose (t=2.07, df=8, p<0.05) and the 6-mg dose (t=2.11, df=8, p<0.05). Similarly, butorphanol at the 6-mg dose produced significant increases in observers' ratings on the Addiction Research Center Inventory LSD scale (t=4.00, df=8, p<0.01). Hydromorphone did not produce significant effects on either of these measures.
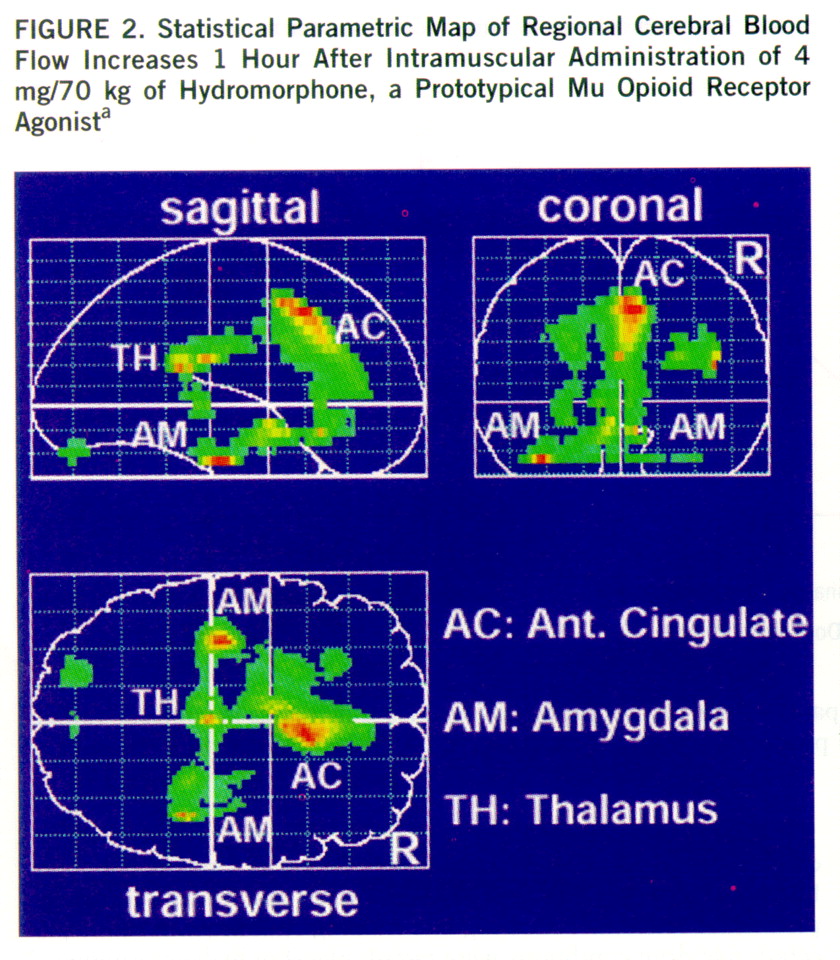
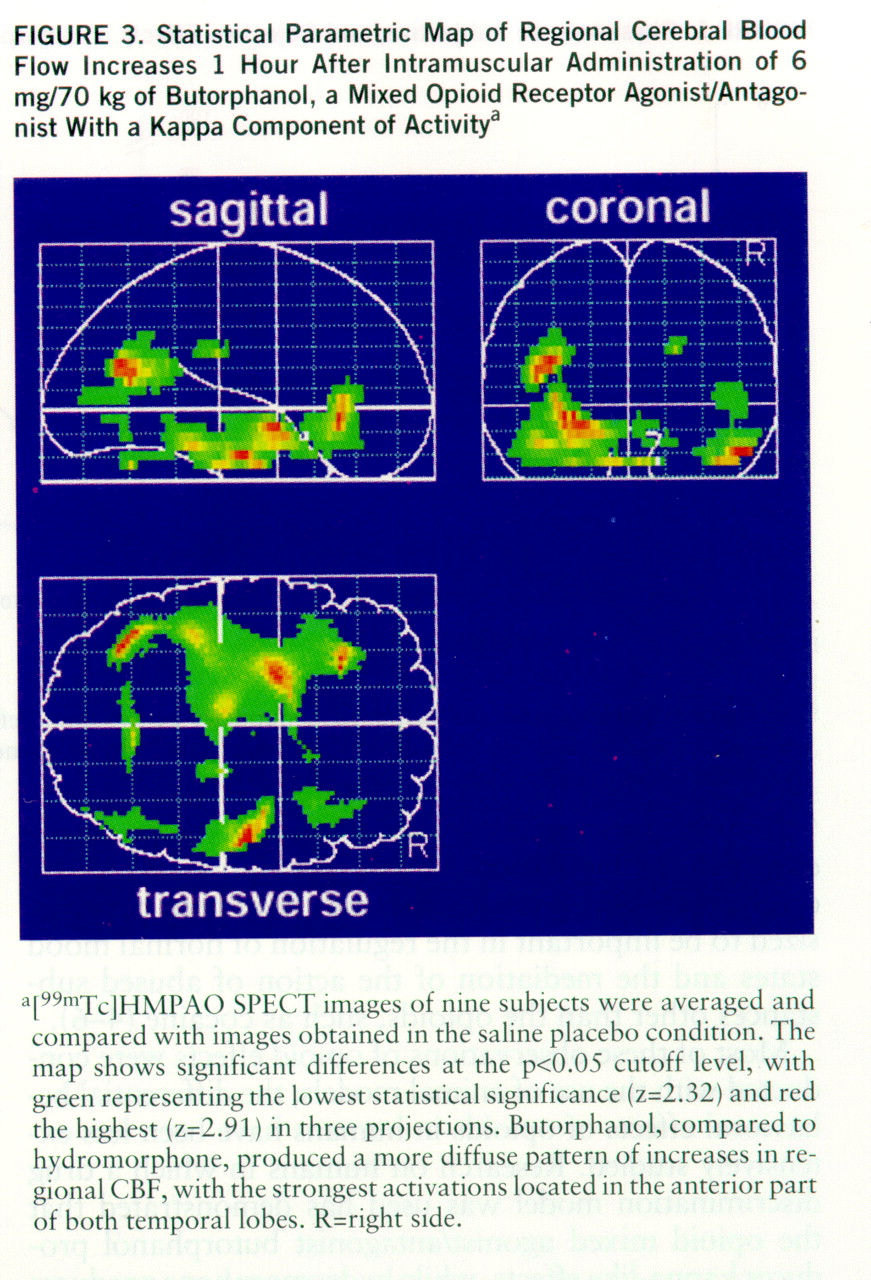
Phase 2: effects on CBF. None of the 27 SPECT scans had to be excluded from the analysis because of artifacts or problems with image quality. The statistical parametric mapping analysis revealed distinct, significant regional CBF increases in the hydromorphone condition as compared with the placebo condition. According to the Talairach-Tournoux atlas, these increases were located in the anterior cingulate cortex, the thalamus, and both amygdalae (
figure 2). Butorphanol produced a more diffuse pattern of increases in regional CBF, with the strongest activations located in the anterior part of both temporal lobes (
figure 3). In both drug conditions the CBF increases were slightly more prominent in the left hemisphere.
DISCUSSION
The results of this study suggest that opioids known to act on different opioid receptor subtypes and to have different patterns of subjective and behavioral effects also produce different patterns of change in regional CBF relative to baseline. We demonstrated increases in regional CBF in the anterior cingulate cortex, the thalamus, and the amygdalae induced by the mu opioid receptor agonist hydromorphone. Butorphanol, which has kappa-like actions, led to more diffuse regional CBF increases relative to baseline, primarily in the temporal lobes. One explanation for the less distinct effects of butorphanol could be its mixed agonist/antagonist properties. The opioid agonist ketazocine would have been a better choice in this study because of its preferential binding to kappa receptors (
19).
In light of the clinical significance of opioid agonist drugs, surprisingly few studies have been undertaken that apply functional neuroimaging methods to assess regional CBF changes associated with behavioral effects of these substances. With the use of serial [
15O]water positron emission tomography (PET) scans in a pain patient, and the same statistical parametric mapping analysis used in the present study, it was found that morphine induced regional CBF increases in the prefrontal and anterior cingulate cortex (
20). Another study, using C
15O
2 PET and the statistical parametric mapping method, reported regional CBF increases in the anterior cingulate cortex and the pericentral cortex induced by administration of the opioid fentanyl (
21). A preliminary SPECT study examining the effects of naltrexone-precipitated opioid withdrawal demonstrated decreases in blood flow in the region of the anterior cingulate of 11 patients maintained on a regimen of buprenorphine; these decreases were strongly correlated with severity of withdrawal symptoms (
22). This last result is particularly interesting, since opioid withdrawal produced an effect on regional CBF that was the opposite of the present study's finding of increases in regional CBF with administration of a mu opioid agonist.
The anterior cingulate cortex is part of the functional circuit of the limbic system and is thought to be involved in the attribution of emotional significance to sensory stimuli (
23). Two other key elements of the limbic system, the thalamus and both amygdalae, showed significant regional CBF increases on statistical parametric mapping analyses in response to the mu opioid agonist hydromorphone. This pattern correlates well with the distribution of mu receptors identified in postmortem pathology studies of human brains (
24,
25). In these studies, high concentrations of mu receptors have been found in the cingulate gyrus, ventral tegmental area, cerebellum, thalamus, and hypothalamus, while kappa receptors have been concentrated in the substantia nigra, parts of the cortex, the amygdala, and also the hypothalamus and cingulate gyrus. Given the limited resolution (6 mm) of the SPECT method, we cannot expect to detect changes in CBF in the smaller of the regions mentioned.
There are important implications of this type of research for substance abuse and pain research. Functional neuroimaging provides a means for assessing the regional changes in CBF associated with drugs of abuse and offers the opportunity to study noninvasively the biological correlates of the reinforcing effects of drugs. Furthermore, CBF effects of novel analgesics can be compared with those of known analgesics. This can provide important information about the locus of activity of analgesics with differing mechanisms of action. Comparisons of the loci of activity associated with euphoric versus analgesic effects may be valuable in aiding the development of analgesics with low liability for abuse.