Risperidone (
1) and clozapine (
2) share some pharmacologic similarities, such as a higher affinity for serotonin 5-HT
2 receptors than dopamine D
2 receptors, but they differ in other pharmacologic properties and side effects (
3). Clozapine produces few or no extrapyramidal symptoms and has been shown to be effective in the management of positive and negative symptoms in chronic and treatment-resistant schizophrenic patients (
4–
8). In controlled short-term studies of patients with chronic schizophrenia, risperidone has been compared with several conventional neuroleptics and shown to be effective against positive and negative symptoms and to be associated with a low incidence of extrapyramidal symptoms (
9–
13).
= We know of only one double-blind comparison of clozapine and risperidone with patients suffering from acute exacerbation of chronic schizophrenia (
14), and it found that clozapine and risperidone were equally effective. However, treatment-resistant schizophrenic patients were not included, and the treatment duration (4 weeks) was short. Risperidone may be a more effective antipsychotic drug than haloperidol (
11) and possibly helpful and effective in treatment-resistant schizophrenia (
11,
12,
15). We therefore conducted a double-blind multicenter study of the clinical efficacy and safety of risperidone and clozapine in 86 inpatients with chronic schizophrenia who were resistant to or intolerant of conventional agents.
METHOD
The study participants were 86 physically healthy men and women. As required by the inclusion criteria, they were aged from 18 years to 65 years (one patient was 17.4 years old) and met the DSM-III-R criteria for chronic schizophrenia. They had previously failed to respond to or were intolerant of at least two different classes of antipsychotic drugs given in appropriate doses for at least 4 weeks each. Treatment resistance and intolerance were determined retrospectively from the patients' files. The primary hospital diagnosis was confirmed by the research psychiatrists on the basis of DSM-III-R criteria. No subject had previously received clozapine. A total score of 60–120 on the Positive and Negative Syndrome Scale (
16) was required.
Excluded from the trial were women of reproductive age who were not using adequate contraception; pregnant or lactating women; patients with epilepsy, other substantial organic or neurologic disease, or clinically relevant abnormal ECGs or laboratory tests; patients with a history of alcohol or drug abuse within the previous 12 months; and patients who had been in trials of investigational new drugs during the preceding 4 weeks.
The study was conducted according to the principles of the Helsinki Pact. Each patient or his or her legal guardian gave written informed consent to participate in the trial. The investigators obtained approval for the study from their respective ethics committees.
Patients were randomly assigned to receive risperidone or clozapine for 8 weeks and were hospitalized during the first 3 weeks of the trial. After the first visit (day –7), all psychotropic and antiparkinsonian drugs were stopped, and most patients entered a 7-day washout period, which was shortened to at least 3 days for the patients with emergent psychotic symptoms. The washout period for the patients treated with depot antipsychotics started on the day they would have received their next depot injection.
At the first visit (day –7), a physical examination was performed, and patients were evaluated with the Positive and Negative Syndrome Scale and the Clinical Global Impression (CGI) scale (
17). The severity of extrapyramidal symptoms was assessed by means of the Extrapyramidal Symptom Rating Scale (
18). The effects of other adverse events on the patients' daily activities were evaluated by means of the
Udvalg for Kliniske Undersϕgelser (UKU) Side Effect Rating Scale (
19). At the second visit (day 0), defined as the baseline for all evaluations, patients were again evaluated with the Positive and Negative Syndrome Scale, the CGI, the Extrapyramidal Symptom Rating Scale, and the UKU scale. Study medications (risperidone or clozapine) were started according to a double-blind, double-dummy protocol.
Psychometric and side effect evaluations were repeated on days 7, 14, 21, 28, 42, and 56. WBC count and differential were determined weekly. Physical health was determined again on day 56. Plasma concentrations of risperidone, 9-hydroxyrisperidone, clozapine, and norclozapine were measured on days 14 and 56 for most of the patients (some centers did not participate in the pharmacokinetic study). The determinations of clozapine and norclozapine concentrations were performed by gas chromatography (nitrogen-phosphorus detector), according to a previously described method (
20) with some modifications. The limit of quantitation was found to be 4 ng/ml for both substances. Risperidone and 9-hydroxyrisperidone in plasma were assayed by a radioimmunoassay procedure as described elsewhere (
21). The plasma drug concentrations were measured 12 hours after the last dose.
The dose of risperidone was increased to 6 mg/day and the dose of clozapine to 300 mg/day during the first 7 days of blind administration, and the doses were maintained at those levels for the next 7 days (until day 14). The titrations and doses of both medications were agreed on by the manufacturers. Doses remained the same or were adjusted on days 14, 28, and 42 according to each patient's response. The dosage ranges were 0–600 mg/day for the clozapine group and 0–12 mg/day for the risperidone group.
Concomitant medications included lorazepam or oxazepam for sleep induction or daytime sedation and biperiden and procyclidine as antiparkinsonian agents for extrapyramidal symptoms. The emergency medication was clothiapine (80 mg/day not more than three times within 14 days).
At each visit, patients were judged a priori to be clinically improved if they had a reduction of 20% or more in total Positive and Negative Syndrome Scale score from baseline. Videotapes of patients' interviews produced by the authors of the scale were used to train investigators and to assess interrater reliability. Each rater was required to achieve an interrater concordance of at least 0.80, as recommended earlier (
16), to participate in the trial. At each visit, patients were also evaluated with the CGI scale to assess their overall status and change from baseline.
The safety of the trial medications was assessed by means of routine physical and neurologic examinations, laboratory tests, determination of body weight, the Extrapyramidal Symptom Rating Scale, and the UKU scale. The Extrapyramidal Symptom Rating Scale was designed to evaluate three types of extrapyramidal symptoms: parkinsonism, dystonia, and dyskinesia.
Measures of efficacy and safety were analyzed according to the intention-to-treat principle. The last-observation-carried-forward technique was used (
22). Parametric data are reported as mean values and standard deviations, determined by analysis of variance and compared by the two-sided Student t test for independent samples. Nonparametric data were compared by means of two-tailed chi-square tests or nonparametric trend analysis. Results were considered to be significant if alpha was <0.05 (
23,
24). In parallel, for the analysis of covariance (ANCOVA), various covariates from the baseline data were tested. Kaplan-Meier survival analysis (
25) was carried out for Positive and Negative Syndrome Scale scores, and the Breslow test (
26) was used to calculate statistical differences between the groups.
As an indicator of the clinical relevance of the treatment results, the analysis included a measure of effect size: d=(mean
1–mean
2)/standard deviation. According to Cohen (
27), a d value of 0.20 indicates a small effect size, 0.50 a medium effect size, and 0.80 a large effect size.
To assess the occurrence of extrapyramidal symptoms, the shift from baseline score to the maximum score on the Extrapyramidal Symptom Rating Scale observed during double-blind treatment was calculated for each patient for each item of the parkinsonism cluster and for the Extrapyramidal Symptom Rating Scale cluster subtotals.
RESULTS
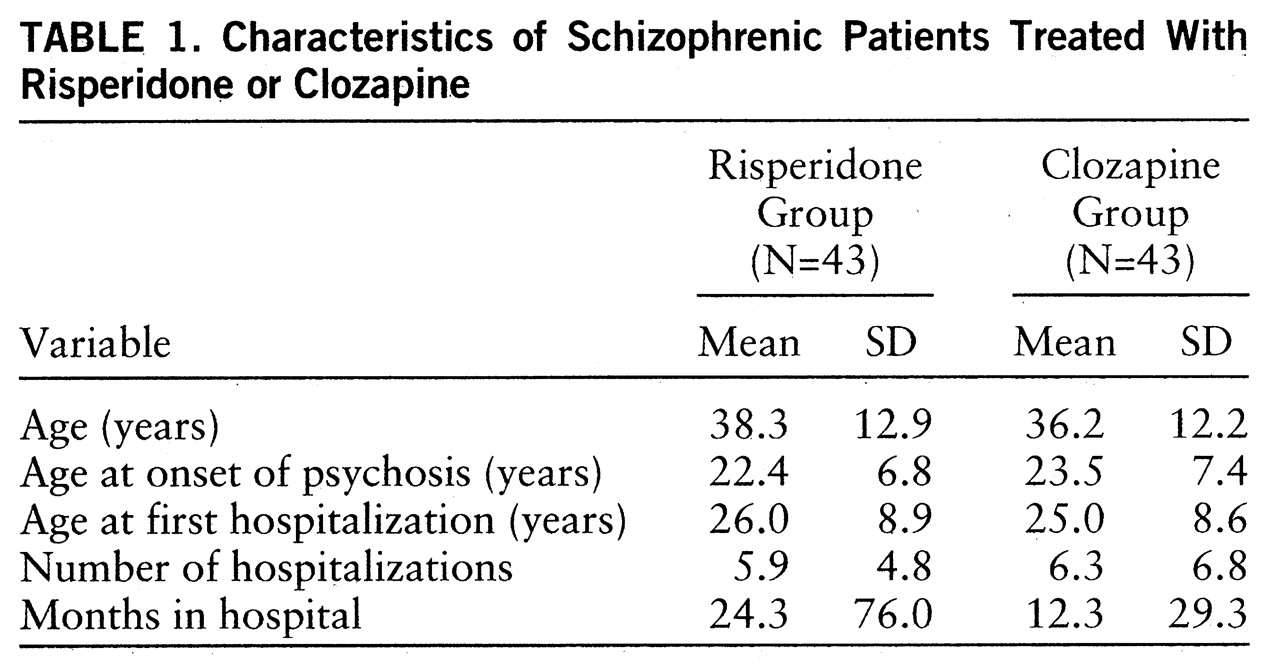
The risperidone group (N=43) consisted of 29 men and 14 women; the distribution of schizophrenia subtypes in the group was 28 paranoid, 10 disorganized, three undifferentiated, and two residual. The clozapine group (N=43) consisted of 32 men and 11 women; the schizophrenia subtypes were 22 paranoid, 14 disorganized, four undifferentiated, and three residual. Background characteristics of the 86 subjects are listed in
table 1. The risperidone group and the clozapine group did not differ significantly in their baseline characteristics, in their mean Positive and Negative Syndrome Scale total scores, negative subscale scores, and general psychopathology subscale scores (
table 2), or in the duration of the washout period. There was a small but significant difference in the positive subscale scores on the Positive and Negative Syndrome Scale at baseline. The washout period was 7 days or longer for 57 patients and less than 7 days for 17 risperidone and 12 clozapine patients.
At the time of enrollment in the study, 64 patients were receiving oral neuroleptics, 12 were receiving depot neuroleptics, and four were receiving both oral and depot neuroleptics; six patients were not receiving neuroleptic treatment. Seven patients in the risperidone group and nine in the clozapine group had been treated with depot neuroleptics before entering the study.
A Kaplan-Meier survival analysis did not show significant differences in time-dependent dropout rates between the two medication groups (not shown). Nine patients in each group dropped out. The reasons for discontinuation were withdrawal of consent (four patients in each group) and adverse events (one patient in each group); insufficient response (four patients in the risperidone group and two in the clozapine group); suicidal thoughts (one patient in the clozapine group); and intercurrent somatic illness (one patient in the clozapine group). Nine patients in the clozapine group and five in the risperidone group received benzodiazepines to control anxiety. One clozapine patient and five risperidone patients were comedicated with benzodiazepines to treat insomnia. Three in each group received antiparkinsonian agents, and two patients in the risperidone group (who also received benzodiazepines) received clothiapine as an emergency medication.
Doses of the two study medications were maintained at initial levels (6 mg/day of risperidone and 300 mg/day of clozapine) throughout the study for approximately two-thirds of the patients. At day 42, the mean doses were 6.4 mg/day of risperidone (range=3–10) and 291.2 mg/day of clozapine (range=150–400), indicating that doses in each group had been adjusted to patients' responses.
Drug Efficacy
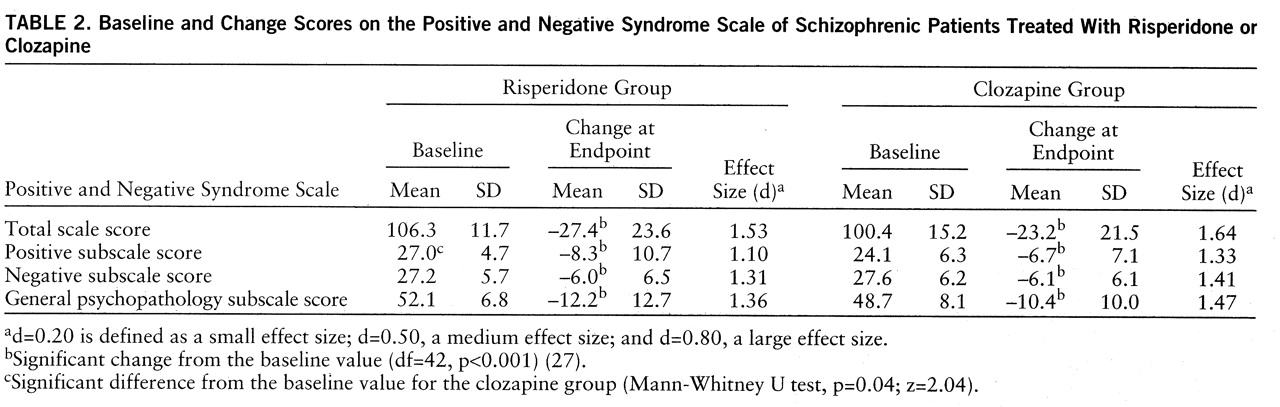
Sixty-seven percent of the risperidone patients (N=29 of 43) and 65% of the clozapine patients (N=28 of 43) were clinically improved at endpoint according to the total Positive and Negative Syndrome Scale scores (
table 2). These differences were not statistically significant, even when adjustment was made for the differences in baseline scores (F=1.07, df=1,83, p=0.30, ANCOVA). The percentages of patients who were clinically improved at different times are shown in
figure 1. In both the risperidone and clozapine groups, the severity of schizophrenic symptoms was significantly ameliorated (
table 2). The large effect sizes demonstrate clinically relevant improvement in both groups at endpoint. Between-group comparisons by means of t tests for independent samples (before and after adjustment for differences at baseline) yielded no statistically significant differences in Positive and Negative Syndrome Scale total and subscale scores. No significant differences between treatments in scores on the CGI scales were found at any time point. At baseline, CGI severity scores were 4.3 in both treatment groups, and at endpoint, the scores were 3.1 and 2.9 in the risperidone and clozapine groups, respectively. CGI change scores at treatment day 7 were 2.4 and 2.7, for risperidone and clozapine, respectively, and at endpoint, 1.9 and 1.7, respectively.
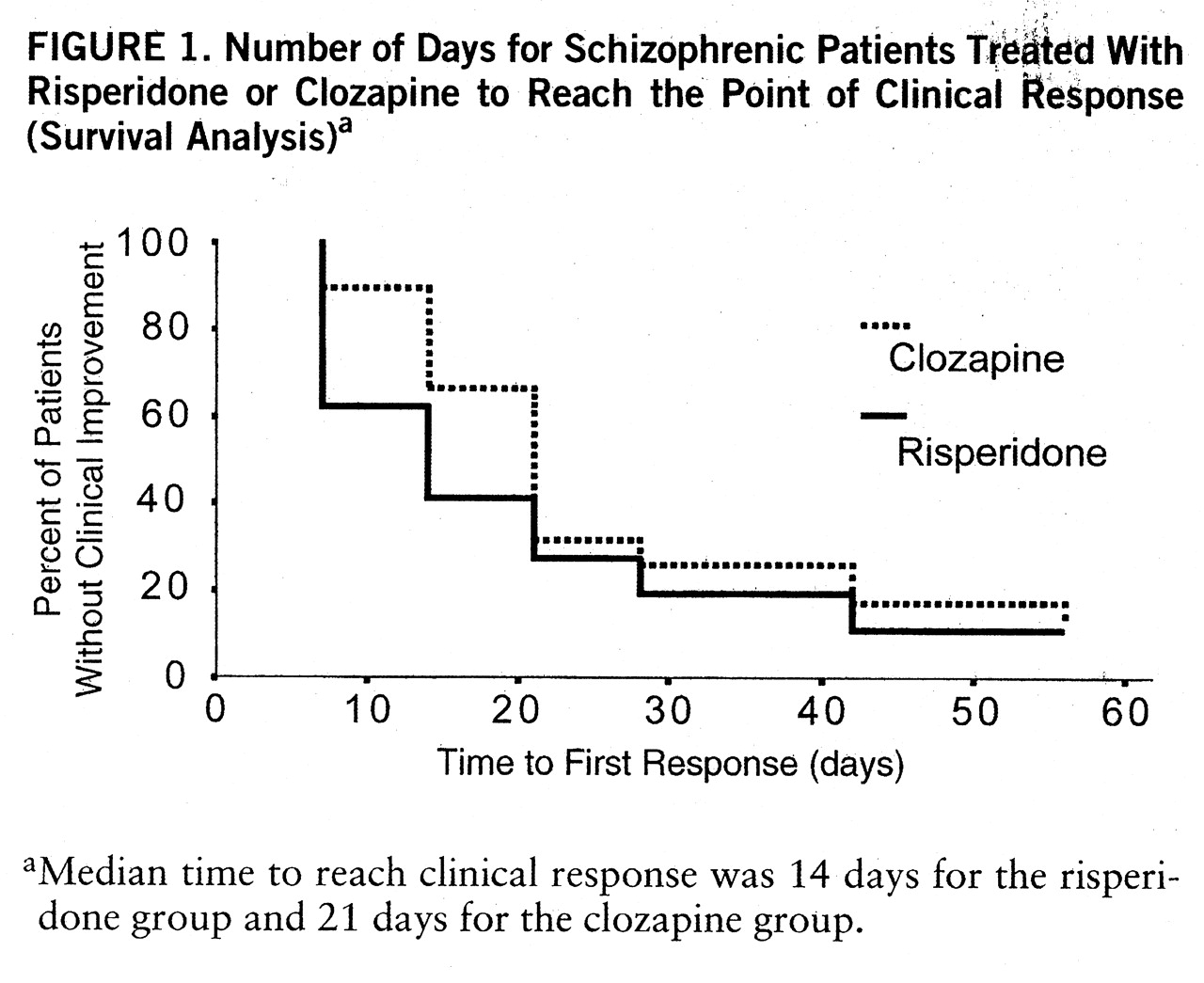
A significantly higher percentage of risperidone patients than clozapine patients had responded to treatment by days 7 and 14 (
figure 1), suggesting that risperidone took effect more quickly than clozapine. Baseline total Positive and Negative Syndrome Scale scores of the responders and nonresponders were then compared. In the risperidone group, there were 15 treatment responders and 23 nonresponders at day 7; their mean baseline total Positive and Negative Syndrome Scale scores were 104.0 (SD=14.7) and 107.6 (SD=9.8), respectively; this difference was not significant. This suggests that the faster onset of action of risperidone was not an artifact of baseline differences. Furthermore, the Kaplan-Meier survival analysis for Positive and Negative Syndrome Scale scores confirmed the faster onset of action of risperidone (Breslow test statistic=4.52, df=1, p=0.03). At each visit after day 14, the percentages of responders in the two treatment groups were not significantly different. There was no evidence of an effect of pretreatment depot antipsychotics on response to clozapine or risperidone: the numbers of patients in each group who had received decanoate therapy (nine in the clozapine group and seven in the risperidone group) were similar, and the duration of the trial (8 weeks) reduces the risk of a bias due to a prolonged effect of this previous treatment. Moreover, the distributions of the patients previously treated by depot antipsychotics among the dropouts (none in either treatment group) and among the treatment responders (six in the clozapine group and four in the clozapine group) were similar.
On day 56, plasma concentrations of clozapine were measured in 28 of 43 patients. The mean concentrations were 292 ng/ml (SD=255, range=102–670) in the nonresponders (N=4) and 281 ng/ml (SD=195, range=87–826) in the responders (N=24). On day 56, six of the 24 responders had plasma concentrations of 350 ng/ml or more, and the other 18 patients, less than 350 ng/ml. There was no evidence of a relationship between clozapine concentration and clinical response. In the risperidone group, there were no differences between the responders and nonresponders when plasma concentrations of risperidone and of its metabolite were considered. On day 56, plasma concentrations of risperidone were measured in 21 of 43 patients. Mean concentrations of risperidone and 9-hydroxyrisperidone were 9.86 ng/ml (SD=13.8) and 35.3 ng/ml (SD=25.5), respectively, in responders (N=15), and 5.09 ng/ml (SD=8.9) and 31.0 ng/ml (SD=18.7), respectively, in nonresponders (N=6). Thus, clinical response was apparently not related to plasma concentrations of risperidone and 9-hydroxyrisperidone or to the sum of both compounds.
Drug Safety
The treatment groups were not significantly different at baseline on any of the Extrapyramidal Symptom Rating Scale cluster scores. The mean cluster scores showed a progressive decrease during the course of the trial under both treatment regimens. There were no significant differences between the groups at endpoint in the mean total Extrapyramidal Symptom Rating Scale scores, the different cluster scores, or the different cluster scores on the parkinsonism subscale. Many patients in both groups were free of extrapyramidal symptoms at endpoint. The percentages of patients in each group who scored 0 on each Extrapyramidal Symptom Rating Scale measure were as follows: total score (parkinsonism plus dystonia plus dyskinesia), 54% of the risperidone group and 37% of the clozapine group; parkinsonism total score, 61% and 37%, respectively; dystonia total score, 95% and 98%; and dyskinesia total score, 84% and 84%. On the CGI, there were scores of 0 on severity of dyskinesia for 88% of the risperidone group and 91% of the clozapine group; on severity of parkinsonism for 67% and 72%, respectively; and on the total questionnaire for 56% and 37%. Antiparkinsonian medication had been stopped at day 7 for all patients except two clozapine-treated patients with mild extrapyramidal symptoms who received such medication before and during the study. Only three patients in each group needed antiparkinsonian medication.
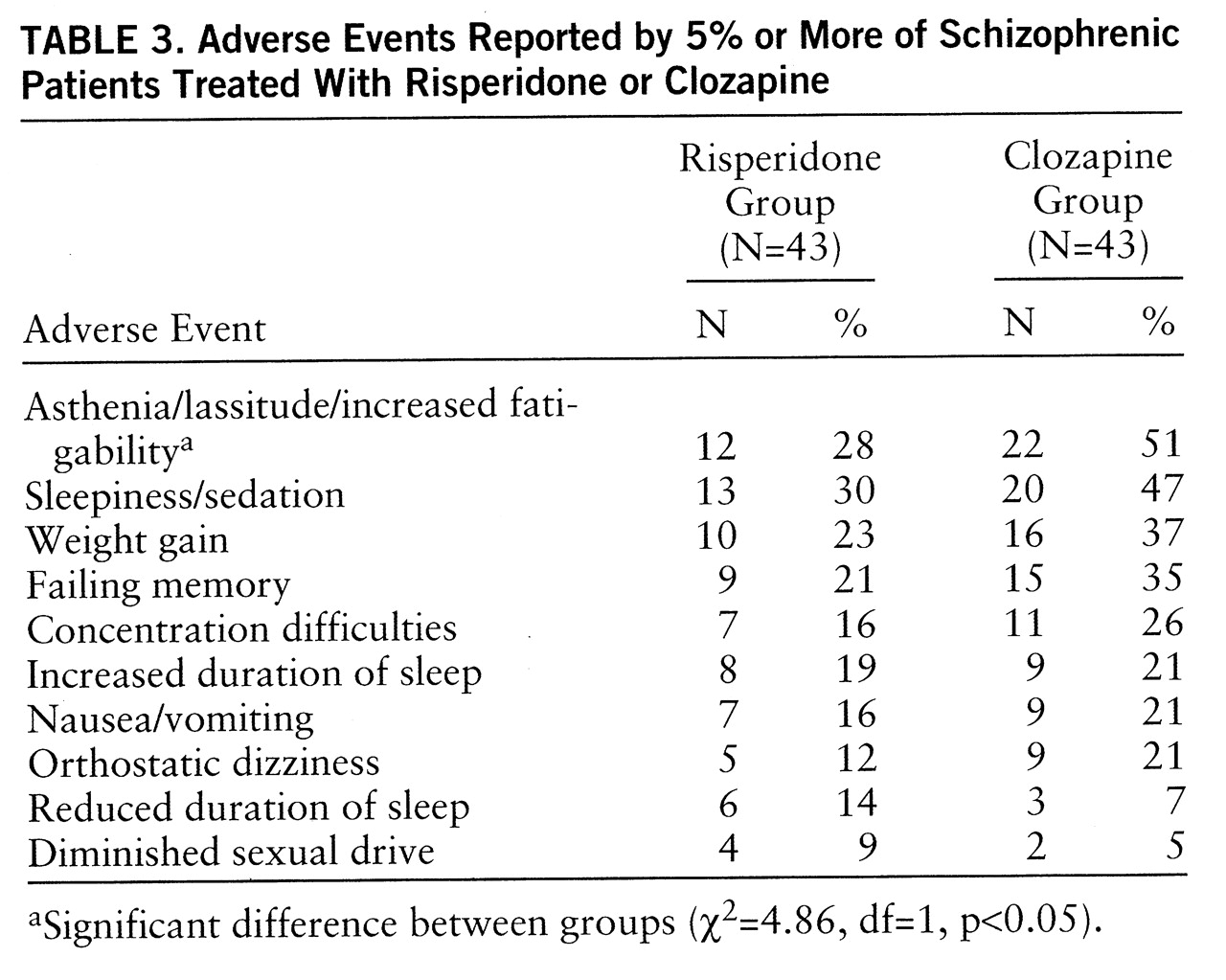
Table 3 shows the adverse events reported by 5% or more of either treatment group during the trial. There were no significant differences among the two groups for these 10 events, except for asthenia/lassitude/increased fatigability, which was reported by more patients in the clozapine group than in the risperidone group. During the titration period, some differences between the groups were observed. On day 0, 10 patients in the clozapine group and 19 in the risperidone group reported asthenia, lassitude, and increased fatigability; on day 7, 18 and 19 patients, respectively, reported these adverse affects. On day 0, sedation and sleepiness were reported by seven patients in the clozapine group and four in the risperidone group; on day 7, these effects were reported by 17 and nine patients, respectively. On day 0, four patients in the clozapine group and none in the risperidone group suffered from ortho~static dizziness; on day 7, 10 and no patients, respectively, suffered this adverse effect.
One patient in the risperidone group, who was suffering from a cold, showed leukopenia (WBC=3500/mm
3) with neutropenia (1820–980/mm
3 neutrophils) (
28) on days 7–9 of treatment. This side effect was reversible after the treatment was stopped. No significant clinical problems with blood pressure, heart rate, or ECG arose, and the two drugs were generally well tolerated by the patients in both treatment groups. In the clozapine group, the mean difference in body weight (2.7 kg) between visit 1 (76.3 kg) and endpoint (79.0 kg) was statistically significant (z=–3.30, p=0.01, Wilcoxon matched-pairs test), but the difference in the risperidone group (1.1 kg) was not (z=–0.61, n.s.).
DISCUSSION
The results of this 8-week trial indicate that both risperidone and clozapine are effective and well-tolerated antipsychotic agents in chronic schizophrenic patients who are intolerant of or resistant to conventional neuroleptics.
In the flexible-dose study design, doses of risperidone and clozapine were adjusted according to the patients' needs. The mean risperidone dose of 6.4 mg/day in this study is within the range of optimal doses defined in previous controlled trials of risperidone in chronic schizophrenic patients (
11–
13). The mean clozapine dose of 291.2 mg/day corresponds to the standard dose used for treatment-resistant patients in Europe but is lower than the doses used in the United States for such a patient population (
29,
30). Several studies show a correlation between plasma clozapine concentrations and clinical response among treatment-resistant schizophrenic patients (
31–
34). Whereas they suggest a threshold value range of 350–420 ng/ml for response to clozapine, it has been recently shown (
30,
35) that lower doses and lower plasma concentrations can be as effective as higher doses in the management of treatment-resistant schizophrenic patients and may cause fewer side effects.
Among the patients in the clozapine subgroup who were included in the pharmacokinetic part of this study, there was no clear evidence of a relationship between plasma clozapine concentration and clinical response. The limited number of subjects does not allow any definitive conclusions; nevertheless, some of the patients who did not respond to clozapine might have improved with higher doses (three out of four had plasma concentrations between 100 and 210 ng/ml).
Plasma concentrations of 9-hydroxyrisperidone were higher than those of risperidone. The sum of levels of risperidone and its metabolite is considered to be the “active moiety”; the pharmacologic profile of 9-hydroxyrisperidone is comparable to that of its parent compound (
36) and may contribute to the overall therapeutic effect. There was, however, no evidence of a relationship between plasma concentration and clinical response. Apparently, no other study up to now has dealt with this question (
37).
Our data suggest that risperidone had a faster onset of action than clozapine, since a significantly greater reduction in symptom severity was observed in the risperidone group than in the clozapine group at days 7 and 14. However, the onset of the therapeutic effect of clozapine can be delayed from 12 weeks to 6–9 months (
8,
38). This indicates that 8 weeks may be too short a period of time to evaluate the overall effectiveness of clozapine and also perhaps of risperidone.
The incidence of extrapyramidal symptoms was similarly low in the two groups, and the severity of these symptoms was generally mild. More patients in the clozapine group than in the risperidone group reported adverse events, but the difference was statistically significant for asthenia/lassitude/fatigability only. The differences in side effects observed during the titration period suggest that some bias may have occurred with regard to the blindness of the trial. Body weight increased in both treatment groups, but the change from baseline was significant only in the clozapine group. This finding corroborates the well-known propensity of clozapine to induce substantial weight gain in a large proportion of patients; this side effect is clinically relevant because it may lead to problems of compliance and involves a potential health risk associated with obesity.
Lieberman et al. (
8) showed that the response to clozapine was better in treatment-intolerant patients than in treatment-resistant patients. Moreover, the only recent study that did not detect a relationship between plasma clozapine concentration and clinical response dealt with either antipsychotic-intolerant or treatment-resistant patients (
6). Patients who do not tolerate standard neuroleptics because of neurologic side effects may possibly respond to lower doses and, accordingly, lower plasma concentrations of clozapine (
39). In the present study, however, treatment-resistant and treatment-intolerant patients could not be clearly differentiated.
The results of this study should be interpreted with caution owing to the limited number of patients. However, when taking into account the large effect size, we found clinically relevant improvement in both groups at endpoint. The efficacy and safety of risperidone and clozapine were found to be similar in two previous comparative trials of the two agents. These were a 4-week double-blind study (
14), in which 39 patients received 4 mg/day (N=19) or 8 mg/day (N=20) of risperidone and 20 received 400 mg/day of clozapine, and an open study (
40), in which 11 schizophrenic patients with neuroleptic-induced supersensitivity psychosis and tardive dyskinesia were treated with risperidone or clozapine for 2–25 months. The results of these two studies and our findings indicate that long-term, controlled comparative trials of risperidone and clozapine in larger, clearly defined, and more homogeneous study groups are needed.
In conclusion, both risperidone and medium doses of clozapine were effective and safe in treatment-resistant or treatment-intolerant patients with chronic schizophrenia. Risperidone, however, appeared to have a faster onset of action than clozapine.