In many respects, the treatment of anorexia nervosa is as challenging today as when the disorder was formally recognized over a century ago. By definition, individuals with anorexia nervosa are seriously underweight but are reluctant to accept the need for weight restoration. The current therapeutic approach relies on a combination of psychological and behavioral techniques often provided in an inpatient or intensive outpatient setting (
1). Although such interventions are generally successful in restoring weight, patients typically remain ambivalent about treatment, and relapse is common (
2,
3). Patients also frequently exhibit symptoms of mood disturbance and of obsessive-compulsive disorder (OCD) that impair psychological functioning. To some degree, such symptoms appear to be a manifestation of malnutrition and improve with weight gain. Nonetheless, obsessionality and mood disturbances often persist even after weight is restored (
3–
5). In addition, a substantial fraction of patients with anorexia nervosa regularly engages in binge eating and/or uses inappropriate methods to avoid weight gain, such as self-induced vomiting; in DSM-IV, such individuals are classified as having anorexia nervosa, binge-purge type.
Pharmacological interventions are of established utility in the treatment of several disorders that symptomatically overlap with anorexia nervosa. Antidepressant medications are, by definition, effective in the treatment of major depression and are clearly useful for bulimia nervosa, an eating disorder closely related to anorexia nervosa (
6). Antidepressant medications that inhibit serotonin reuptake (SSRIs) are beneficial in OCD (
7). Several anecdotal reports have suggested that SSRIs might be valuable in the treatment of underweight patients with anorexia nervosa (
8,
9). Therefore, the present study was conducted to determine whether, when combined with a structured inpatient program for anorexia nervosa, the SSRI fluoxetine was associated with greater weight gain and improved psychological functioning compared to placebo.
METHOD
Subjects
Subjects were women between the ages of 16 and 45 years receiving inpatient treatment for anorexia nervosa. Subjects were required to meet DSM-IV criteria A–C for anorexia nervosa and to weigh less than 80% of ideal body weight (according to the 1959 Metropolitan Life Insurance Tables [10]). Subjects were excluded if they 1) were medically unstable; 2) reported a past allergy to fluoxetine; 3) met criteria for alcohol or drug dependence in the last 6 months; 4) met criteria for bipolar illness or for psychotic disorder, current or lifetime; or 5) met criteria for OCD with onset before that of anorexia nervosa. After a complete description of the study was given to the subjects, written informed consent was obtained, and, for those subjects who were under the age of 18, parental consent was also obtained.
Thirty-three subjects entered the study and were randomly assigned to fluoxetine or placebo, but data from two patients were not analyzed. One of these patients withdrew from treatment and left the hospital 5 days after beginning study medication; the other patient was found to have undetectable levels of fluoxetine in her blood, despite being randomly assigned to medication. Self-report data from one additional patient were not analyzed because she was an unreliable reporter of symptoms. Of the 31 patients included, 23 were amenorrheic, five were taking birth control pills and were menstruating regularly, and three reported irregular menses. These three patients were included despite the absence of amenorrhea because they were substantially underweight (76%, 73%, and 78% of ideal body weight) and met all other diagnostic criteria for anorexia nervosa.
Procedure
Patients were treated on an inpatient research unit. They were seen three to five times weekly in individual psychotherapy that included both supportive and cognitive-behavioral elements. They also attended a variety of group treatment sessions each week and were seen with their family if available. In addition, all patients participated in a structured behavioral treatment program aimed at normalizing eating behavior and weight. In the weight gain phase of this program, which began shortly after admission, patients were given a prescribed number of calories and were expected to gain at least 1.5 lb. weekly until reaching 90% of ideal body weight. Weight was measured three times a week, and if sufficient weight was not gained, patients were prescribed additional calories and remained on bed rest until weight gain resumed. The second phase of the program aimed to prepare patients for discharge through focusing on maintenance of weight and on normalizing eating behavior.
Random assignment to fluoxetine or matching placebo occurred after the patient was medically stable (as determined by physical examination, routine laboratory assessments, and ECG) and had begun the weight gain program. In addition, subjects were not randomized until they had reached 65% of ideal body weight. Randomization was stratified on the basis of DSM-IV subtype (restricting versus binge/purge) and on the presence of current major depression. Neither patient nor staff was informed of medication assignment. Fluoxetine was initiated at a dose of 20 mg/day and increased to 60 mg/day over 1 week. This dose was maintained until the conclusion of the trial unless the subject reported intolerable side effects, in which case the dose was decreased or the medication discontinued. Each patient continued in the study until her weight reached 90% of ideal body weight and remained at or above that level for 1 week, or for a maximum of 7 weeks.
Measures
Diagnoses were established before randomization through use of the Structured Clinical Interview for DSM-III-R (
11). Patients were weighed three times weekly in the morning, after voiding, wearing only underwear. Patients were assessed weekly by the clinical staff with the Anorexic Behavior Scale (
12) and a 7-point Clinical Global Impression (CGI). The patients completed the Beck Depression Inventory (
13) weekly. In addition, at the beginning, midpoint (week 4), and end of the trial, patients completed the following self-report instruments: the Body Shape Questionnaire (
14), the Eating Attitudes Test (
15), and the SCL-90 (
16). At the beginning and end of the trial, a research assistant interviewed the patients with the Yale-Brown-Cornell Eating Disorder Scale (
17). Plasma samples were obtained at week 4 and at termination for determination of fluoxetine and norfluoxetine levels.
Data Analysis
For measures obtained at both randomization and termination, change was examined by using paired t tests. The status at termination of patients assigned to fluoxetine was compared to that of patients assigned to placebo by using analyses of covariance (ANCOVAs), with the status at randomization as the covariate. For measures that were available only at termination, fluoxetine- and placebo-treated groups were compared by using analysis of variance (AN~OVA). Rate of change in body weight was assessed by dividing the change in percent of ideal body weight during the trial for each patient by the number of days she was in the trial. Analyses were conducted through use of SPSS-PC.
RESULTS
The mean age of the group was 26.2 years (SD=7.4); the patients had had anorexia nervosa for 8.0 years (SD=5.8). The average weight at time of randomization was 92.0 lb. (SD=9.8), equivalent to a mean of 72.5% of ideal body weight (SD=5.3%), and the average body mass index was 15.0 kg/m2 (SD=4.2). There were no significant differences in the clinical characteristics of the fluoxetine and placebo groups at the time of randomization, except for a small difference in mean age (fluoxetine group: 29.1 years, SD=7.2; placebo group: 23.4 years, SD=6.4) (t=2.35, df=29, p<0.03); age was not related to clinical outcome on any measure.
The average duration of medication treatment was 36.1 days (SD=14.1) for those receiving fluoxetine and 37.4 days (SD=13.8) for those receiving placebo (t=0.25, df=29, n.s.). The mean dose at termination was 56.0 mg/day (SD=11.2) for the fluoxetine group and the equivalent of 58.7 mg/day (SD=5.0) for the placebo group (t=0.89, df=29, n.s.). The mean plasma levels for those receiving medication were as follows: fluoxetine, 330 ng/ml (SD=181), and norfluoxetine, 254 ng/ml (SD=116).
In general, fluoxetine was very well tolerated. Four (27%) of the 15 patients receiving fluoxetine and four (25%) of the patients receiving placebo terminated the trial before reaching 90% of ideal body weight or completing 7 weeks of the study. In the medication group, two patients were discharged prematurely at their request, against medical advice, and two were withdrawn because of persistently severe or worsening depression. In the placebo group, two patients were discharged prematurely at their request, against medical advice; one withdrew because of persistent severe anxiety, and one withdrew because of recurrent headaches. Two other patients receiving medication complained of side effects but were able to complete the entire trial with a decreased dose of fluoxetine. One patient's dose was reduced to 40 mg/day because of insomnia and agitation, and one patient's dose was reduced to 20 mg/day because of blurred vision.
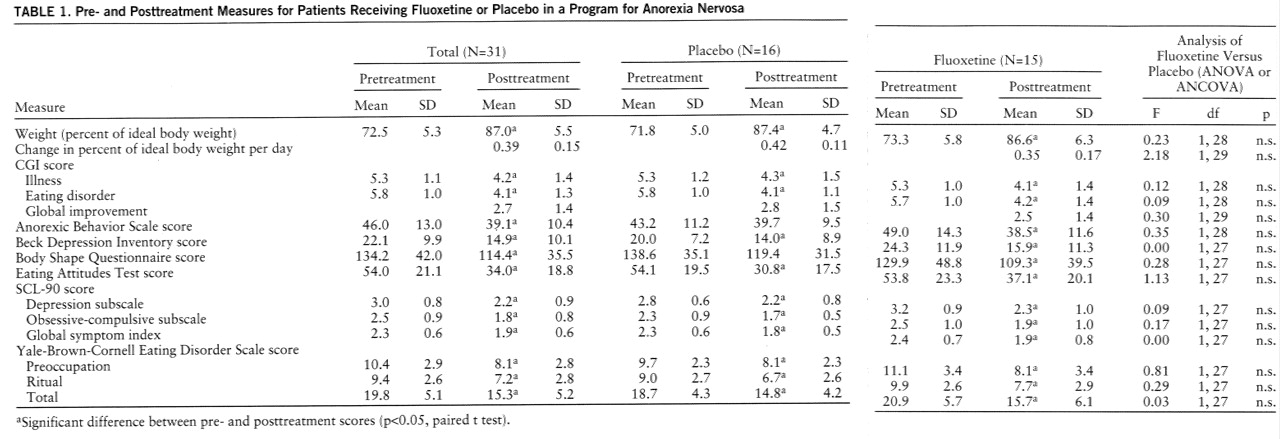
Table 1 presents information on patient status at the beginning and end of the medication trial. Patients in both fluoxetine and placebo groups showed statistically significant improvement on virtually all measures. However, there were no indications of a difference between the fluoxetine and placebo groups.
No difference between the fluoxetine and placebo groups was observed among the 17 patients with current major depression. Similarly, there were no indications of a difference between medication and placebo among the 12 patients with the restricting subtype or among the 19 patients with binge/purge subtype.
DISCUSSION
Compared to placebo, fluoxetine conferred no additional benefit to the inpatient treatment of underweight patients with anorexia nervosa. This result is consistent with three previous controlled trials of antidepressant medication in this setting, which found that the tricyclic antidepressants amitriptyline and clomipramine were of limited, if any, value (
18–
20). Because patients with anorexia nervosa frequently exhibit symptoms of depression and of OCD that are known to respond to antidepressants, the consistent failure of these pharmacological interventions in anorexia nervosa is puzzling. Several explanations may be offered.
First, a number of methodological limitations restrict the power of the studies thus far conducted to detect differences between medication and placebo. The trials of antidepressant medication have been carried out with small or modest sample sizes. The study of clomipramine had only eight patients in the medication group (
18), and the amitriptyline studies had 11 and 23 (
19,
20) patients in the medication group. The group size of the current study provided a power of approximately 75% of detecting a statistically large effect of medication, and it is therefore possible that an effect of modest size was missed. On the other hand, the effect sizes actually observed were small, suggesting that it is unlikely that fluoxetine has a major impact on the treatment of anorexia nervosa as provided in this study.
Another potential concern is the dose of antidepressant medication. In the trials of tricyclic antidepressants, the dose was modest, even allowing for the low weights of the patients. The trial of clomipramine used only 50 mg/day, one trial of amitriptyline used an average of 2.8 mg/kg/day (
20), and the other used a maximum of 160 mg/day (
19). Inadequate dose of medication would not appear to be a problem in the current study; the average dose was 56 mg/day, which is in the range established to be effective in the treatment of normal-weight patients with bulimia nervosa and of patients with OCD and is greater than the usual antidepressant dose of 20 mg/day (
21).
Another significant methodological issue in the current and the previous trials of antidepressant medication in anorexia nervosa is that the medication was provided as an addition to an established treatment program. Thus, these studies have not asked whether antidepressant medication is superior to placebo as the only treatment; rather, these studies have attempted to determine whether it is useful to add medication to a structured psychological and behavioral program that clearly provides benefit. In the current study, the placebo group showed significant improvement in weight and in virtually all measures of psychopathology, including depression and obsessionality. Therefore, the opportunity to observe additional benefit from fluoxetine may have been limited. On the other hand, it should be noted that the patients were clinically symptomatic at the conclusion of the trial, so that an absolute “ceiling” was not reached; there was certainly still room for clinical improvement.
There was also no indication of a difference between fluoxetine and placebo among the patients who were depressed or among those with bulimic symptoms. Both of these findings are at least somewhat surprising, in light of the established utility of fluoxetine in these conditions in individuals without anorexia nervosa. However, the very small sizes of these subgroups severely limited the power of this study to detect a medication effect.
Although the controlled trials of antidepressant medication in anorexia nervosa are few and limited by the methodological issues just discussed, the consistent failure to observe a significant difference between medication and placebo suggests that antidepressant medication is of little, if any, benefit in the treatment of this disorder. Neurochemical disturbances have been documented in anorexia nervosa, including the level of the major metabolite of serotonin, 5-hydroxyindoleacetic acid, which changes as weight improves (
22). It is conceivable that the presence of such neurochemical disturbances interferes with the effects of fluoxetine that underlie its clinical impact. It is of interest that Kaye et al. (
23) have recently reported that fluoxetine was superior to placebo in preventing relapse among patients with anorexia nervosa; in that trial, medication was not initiated until after the patients' weights had been restored to near-normal levels.
In summary, the current study found no evidence that the addition of fluoxetine to a structured inpatient program significantly improved clinical outcome of patients with anorexia nervosa. The study was of modest size, and it remains possible that fluoxetine might be of benefit to a subgroup of patients with this illness.