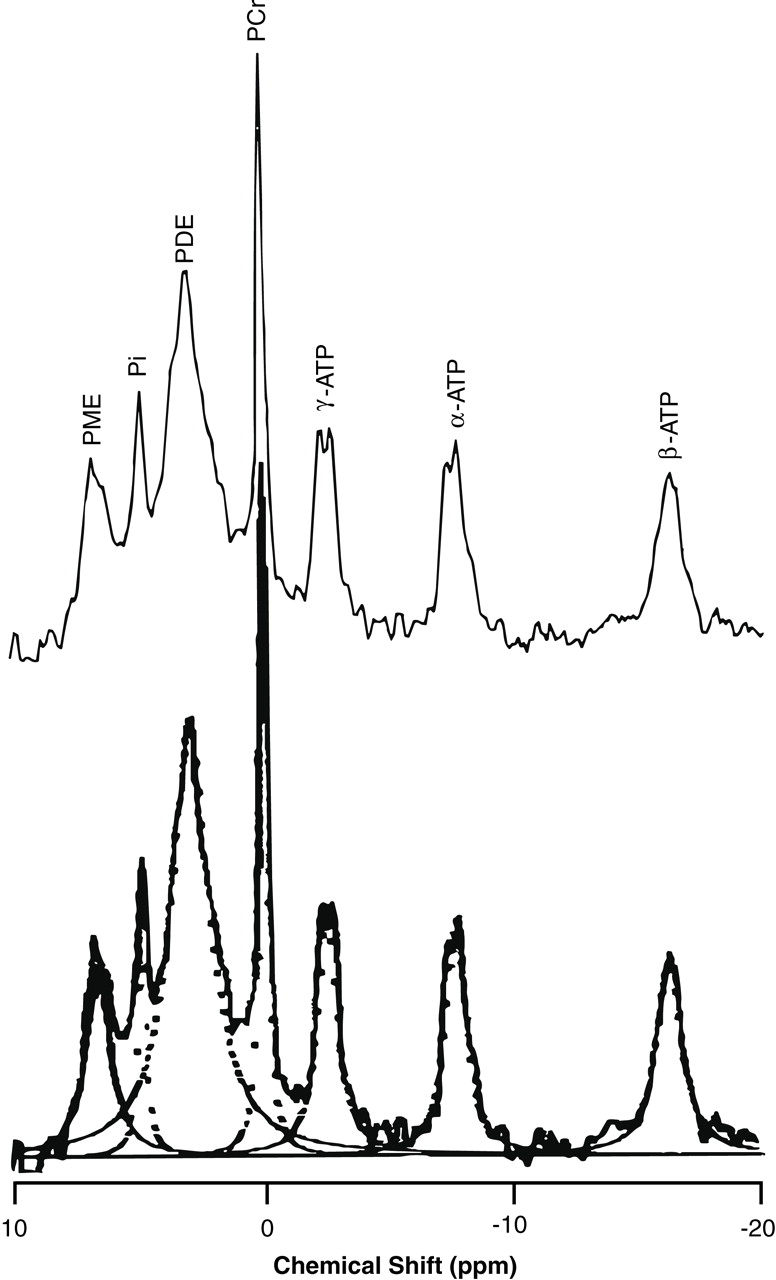
In this study,
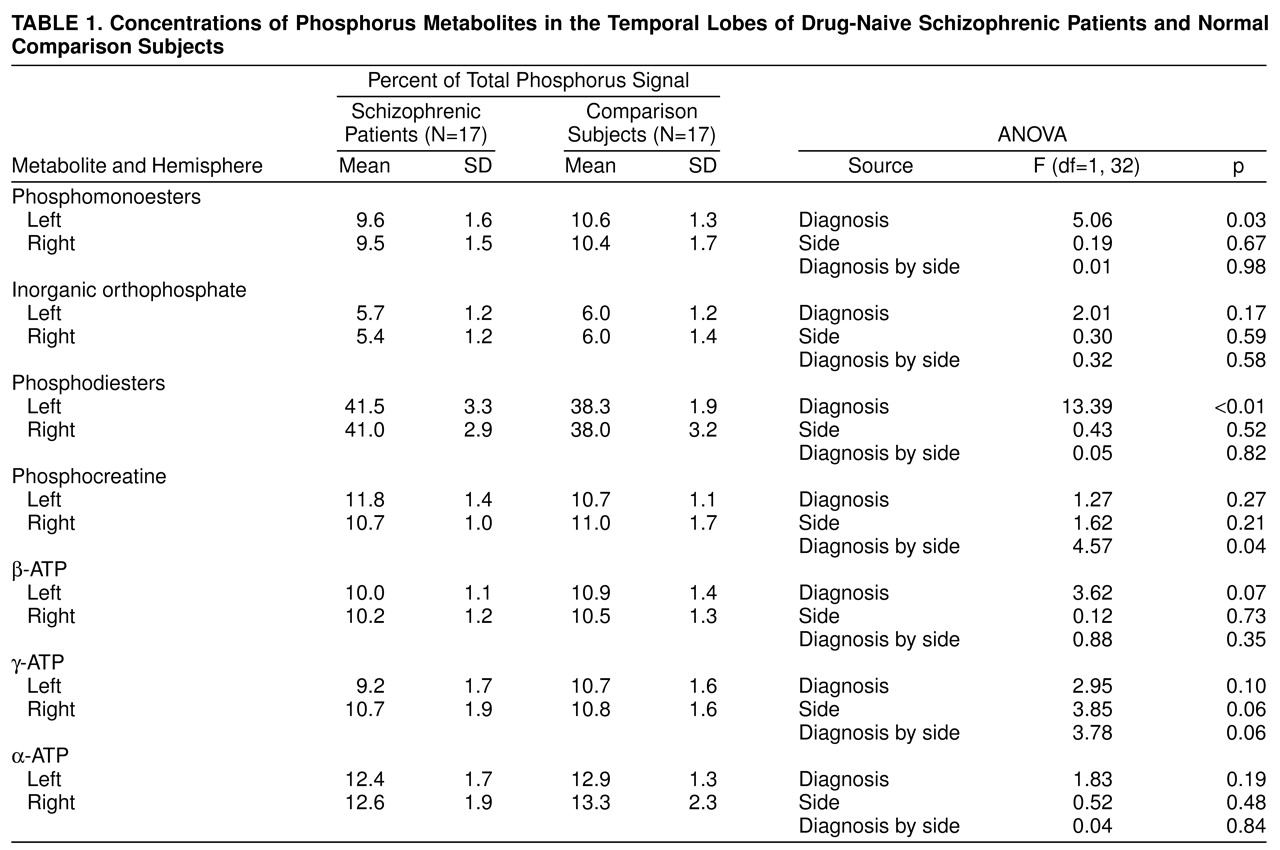
31P MRS detected an elevation of phosphodiesters and reduction of phosphomonoesters in the temporal lobes of drug-naive schizophrenic patients compared with healthy subjects. These results are consistent with previous observations reported in the prefrontal cortex of schizophrenic patients
(2,
3). Pettegrew et al.
(2) have speculated that decreased phosphomonoesters and increased phosphodiesters in the frontal lobes of schizophrenic patients may reflect decreased synthesis and increased breakdown of membrane phospholipids. However, the interpretation of the findings is not easy, as they previously suggested. Decreased phosphomonoesters resonance in this study may imply a reduction in freely mobile phosphomonoesters (phosphocholine, phosphoethanolamine) or less mobile molecules (including phosphorylated proteins) or both
(1). Reduced synthesis of membrane phospholipids is one of these possibilities. The phosphodiester resonance in
31P MRS in vivo is believed to be derived from mobile phosphodiester moieties (small membrane phospholipid structures such as micelles and vesicles) and breakdown products
(1,
12). Phosphodiesters are more concentrated in white than in the gray matter
(13). Therefore, the increase in phosphodiesters could have resulted from increased mobile phosphodiester moieties including glycerophosphocholine and glycerophosphoethanolamine, as well as small membrane phospholipid structures, or it could have been a reflection of a decreased ratio of gray-to-white matter volume in the volume of interest
(14). Which phosphodiester components contribute to the elevation of phosphodiester resonance could be distinguished by using
1H-decoupled
31P MRS. A preliminary study has shown that membrane or mobile phospholipids are increased in the frontal lobes of chronically medicated schizophrenic patients
(6). On the other hand, elevations of glycerophosphocholine and glycerophosphoethanolamine concentrations have been demonstrated in the parietal lobes of young medicated schizophrenic patients compared with elderly schizophrenic patients and healthy subjects
(15). Although definitive determination of the origins of decreased phosphomonoesters and increased phosphodiesters is difficult, the disturbed membrane phospholipid metabolism may not be restricted to the frontal lobe in the manner of the gray matter volume reduction observed in schizophrenic patients
(16).
The level of phosphocreatine was increased in the left temporal lobe in schizophrenic patients. Phosphocreatine is known to be rapidly transformed to ATP when ATP is consumed by neuronal activity
(17). An increased percentage of phosphocreatine may imply reduced ATP utilization in the left temporal lobe of drug-naive schizophrenic patients. This asymmetric abnormality in energy metabolism agrees with left-sided functional impairments observed in the temporal lobes of schizophrenic patients with single photon emission computed tomography
(18).
Several methodologic limitations need to be addressed. The phosphorus metabolites were analyzed without correction for multiple comparison because of the small group size and the exploratory nature of this study, in spite of the increased risk of a type I error. Our curve-fitting method may have a drawback in that all seven metabolites were modeled as single spectral peaks, which could influence the results. Other methodologic limitations inherent in MRS procedures have been outlined in previous reports
(8,
19). Further studies, in a larger group and with more sophisticated in vivo MRS techniques, will be needed to confirm our preliminary findings.