Schizophrenia is a complex illness that is characterized by significant impairment of cognitive, social, and psychological functioning. It has been suggested that defects in one or more basic neurophysiological mechanisms might account for such symptoms. One such model, proposed by British neurophysiologists, is that schizophrenia patients have a deficit in filtering or gating the response to extraneous sensory stimuli; hence, they are flooded by sensory input that they then have trouble organizing
(1). In recent years, several methods have been used to investigate this putative deficit in inhibitory neuronal processing. These have included visual backward masking
(2), prepulse inhibition of the acoustic startle response
(3), and P50 auditory sensory gating
(4–
12). When treated with first-generation or typical neuroleptics, patients with schizophrenia still have profound cognitive, social, and psychological dysfunctions. Typical neuroleptic medications, even in doses high enough to cause extrapyramidal symptoms requiring antiparkinsonian medication, fail to ameliorate most neurophysiological deficits in schizophrenia, including P50 auditory gating deficits
(8), visual backward masking
(13), and prepulse inhibition of the acoustic startle response
(3,
14).
The assessment of sensory gating by the P50 response to paired auditory stimuli is similar to the method used by Eccles
(15) to demonstrate the existence of inhibitory neuronal pathways in animal models. The first stimulus of the pair activates both excitatory and inhibitory mechanisms. The initial excitatory response occurs before the inhibitory response can develop and thus reflects the capacity of the neuronal system under study to respond in the absence of any inhibition. The second stimulus of the pair elicits a diminished response because the inhibitory mechanism activated by the first stimulus interferes with the excitatory response to this second stimulus. The decrement of the second response is thus the test of the strength of the inhibitory mechanisms activated or conditioned during the first responses. The decrement in response is expressed as the percentage ratio of the second or test amplitude to the first or conditioning amplitude.
Most schizophrenia patients have P50 ratios over 50%, often 90% or more
(16). In contrast, most normal subjects have P50 ratios under 40%. This P50 gating deficit appears to be a genetic trait in schizophrenia patients that is found in half their first-degree relatives
(16–
18). Inhibition of the P50 response to a stimulus repeated 500 msec later does not imply that the second stimulus is not heard. Auditory stimuli are processed in many parallel neuronal systems, not all of which are inhibited with the same parameters as P50. The auditory cortex obviously processes trains of stimuli such as speech or music at much faster intervals than 500 msec. However, inhibition of the P50 response in the limbic system results in less attention to common repetitive stimuli, permitting attention to be focused on critical stimuli. However, if the first or second stimulus is novel, then inhibition or gating of the response may decrease. Because they have a deficit in filtering repetitive or extraneous input, patients with schizophrenia are thus less able to relegate repetitive stimuli to a low level of importance and more likely to be distracted by their environment. Accordingly, decreased P50 inhibition in schizophrenia is correlated with decreased sustained attention on neuropsychological testing
(19).
Typical neuroleptic treatment does not ameliorate this deficit
(8,
20). However, recent studies of the atypical antipsychotic medications suggest effects on P50 gating. Clozapine treatment improved P50 gating in patients who responded to clozapine; their P50 gating had been abnormal during previous treatment with typical neuroleptics
(21). In a subsequent follow-up over a 5–27-month period of observation, clozapine’s amelioration of the P50 auditory gaiting deficit was stable
(22). Furthermore, the improvement in P50 gating was paralleled by a corresponding decrease in symptoms as rated by the Brief Psychiatric Rating Scale
(23). This was the first study in which any antipsychotic medication was associated with improvement in P50 gating in schizophrenia patients.
Light et al.
(24) also found a significant enhancement of P50 auditory gating in medicated schizophrenia outpatients treated with atypical antipsychotics. Post hoc division of their subjects treated with atypical antipsychotics suggested that this improvement appeared to be mainly based on the effects of clozapine, and to a lesser degree olanzapine. Subjects receiving risperidone continued to have sensory gating in the range seen with patients receiving typical neuroleptics. Yee et al.
(25) reported that risperidone did not have a significant effect on suppression of the P50 ratio in patients with recent-onset schizophrenia, although they did find some improvement in the suppression of the response to the second click as compared with subjects receiving typical neuroleptics. Arango et al.
(26) in a recent study that compared haloperidol and olanzapine found no significant differences on P50 auditory gating—neither was effective.
Atypical medications differ from each other, as well as from typical antipsychotics, in their occupancy of various catecholaminergic and serotonergic receptors at therapeutic doses
(27,
28), and there are significant differences in their effect on behavioral outcome measures such as negative symptoms, cognitive dysfunction, and mood stabilization (for review, see reference
29). Their different effects on sensory gating have not been well characterized. In this study we compare sensory gating in subjects receiving different neuroleptic medications, both typical and atypical, with patients receiving no medication and healthy comparison subjects.
Method
Subjects
All subjects responded to advertisements placed at the University of Colorado Health Science Center and Denver VA Medical Center and associated clinics. The healthy subjects (N=177) had been recruited for genetic studies, and their results have been previously reported
(30). Of the schizophrenia patients (N=132), 88 were receiving atypical antipsychotics, 34 were being treated with typical antipsychotics, and 10 had been unmedicated for at least 2 months prior to testing. Data on most of the subjects receiving clozapine have previously been reported
(21,
22). The majority of subjects receiving other atypical neuroleptics were recruited specifically for this study. Patients being treated with typical neuroleptics and the unmedicated patients were recruited primarily for earlier genetic studies
(30). All subjects signed informed consent.
The healthy subjects had no personal or family history of an axis I psychotic disorder, and none was taking neuropsychiatric medication. All patients fulfilled DSM-IV criteria for schizophrenia as assessed by interview by the treating or research psychiatrist, review of medical records, or the SCID. Subjects were excluded if they had abused drugs or alcohol in the past 90 days as confirmed by clinical assessment and urine toxicology if indicated. All patients were clinically stable and had been receiving their current medications and doses for a minimum of 1 month. Their classification into a treatment group depended on their primary treatment medication. Some patients treated with atypical neuroleptics were also taking low doses of typical antipsychotics or lithium; many subjects were also taking antidepressants. Since patients had been recruited over a 12-year period from a variety of clinical settings, there were no systematic decision criteria for which medication, particularly which atypical antipsychotic, subjects were prescribed.
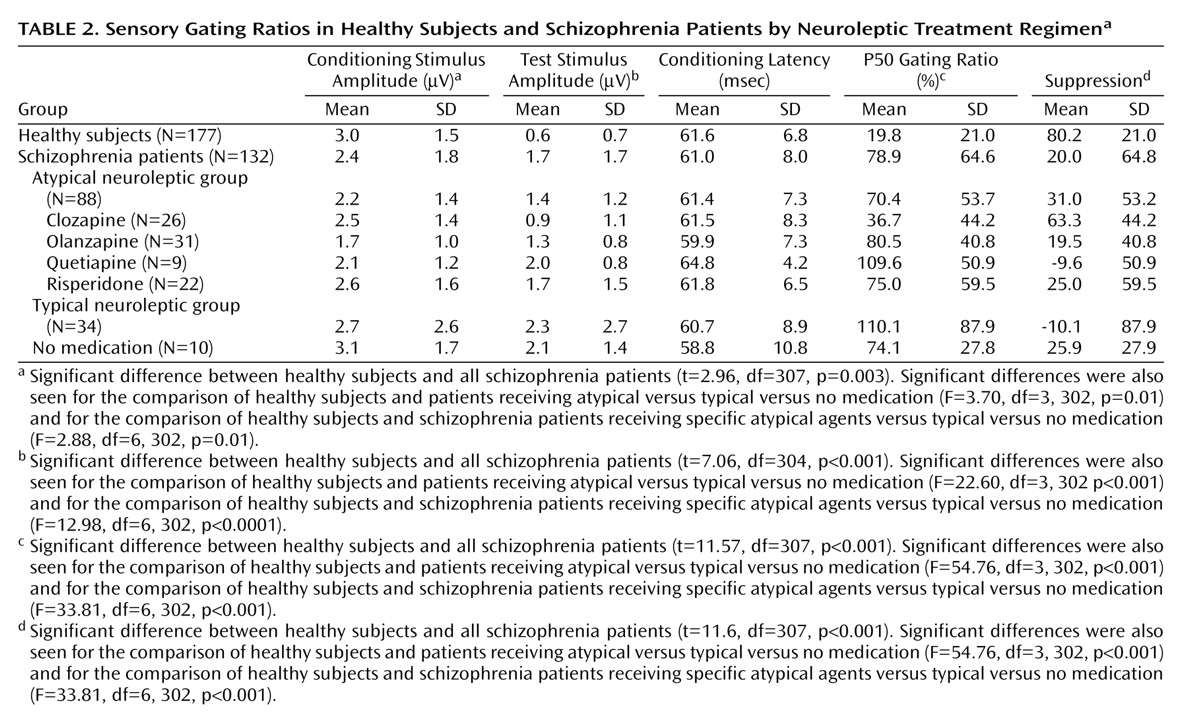
As seen in
Table 1, 114 healthy subjects were female, and 46 of the schizophrenia subjects were female. Subjects ranged in age from 17 years to 69 years (mean=38.4, SD=10.3). There were no significant differences between the mean ages of male and female subjects, and no age differences between the treatment groups. There were no significant gender differences on any of the independent measures.
Of the schizophrenia patients being treated with atypical neuroleptics, 31 were receiving olanzapine, 26 were receiving clozapine, 22 were receiving risperidone, and nine were receiving quetiapine. Ten subjects were unmedicated, primarily because of long-term noncompliance with their treatment regimen. They were a group of subjects who functioned reasonably well without neuroleptic treatment. Thirty-four patients were being treated with a wide variety of traditional neuroleptics that included haloperidol, fluphenazine, and loxapine.
Electrophysiological Recordings
Subjects were supine and awake, with eyes fixed on a specific spot on the ceiling or wall in front of him/her. Recordings were obtained with a gold disc electrode affixed to the vertex and referenced to one ear. Electrode resistance was less than 10 kΩ . Electroencephalogram (EEG) activity was amplified 20,000 times with filters (–50%, 6 dB) at 1.0 and 300 Hz (Grass Instruments, Quincy, Mass.). Data were acquired at a 1000 Hz digitization rate. The electro-oculogram (EOG) recording from the superior orbital ridge referenced to the lateral canthus was also obtained. EEG and EOG records were monitored during the recording by a technician. Individual trials were rejected when EEG or EOG voltage was greater than ±35 μV, indicative of excessive muscle activity, eye movement, or other artifact.
A 0.04-msec duration square wave pulse was amplified in the 20–12,000 Hz bandwidth and delivered through headphones. Stimuli were presented as a series of auditory click pairs. The interval between click pairs was 10 seconds. At least three sets of averaged evoked responses to 16 pairs of stimuli were obtained from subjects at an interval of 500 msec between the conditioning stimulus (first click) and test stimulus (second click). Grand averages were computed for each individual, with a mean of 71.6 (SD=32.2) (of a total presented of mean=115.2, SD=54.9) individual trials for patients with schizophrenia and a mean of 77.6 (SD=9.9) (of a total presented of mean=108.4, SD=32.6) individual trials for the healthy subjects. Neither the number of trials presented nor the number of trials used in the grand averages significantly differed between the groups.
Response Analysis
Averaged evoked responses were analyzed by computer using a previously described algorithm
(31). Grand averages of evoked responses of each set were digitally filtered with a recursive high-pass filter (A=0.95)
(32) and a 7-point low-pass smoothing routine. The filter was applied in both the forward and reverse direction to preserve waveform latency. This filter has only a –3 dB band pass attenuation at 10 Hz and 110 Hz and thus preserves a broader range of frequencies than were studied in other recent reports that used narrower band-pass windows with great rolloff
(33). Inclusion of lower frequencies has been associated with less distortion of the P50 wave and greater detection of suppression in the paired stimulus paradigm
(34–
36). The computer then selected the most positive peak between 40 and 90 msec after the conditioning stimulus. Amplitude was measured relative to the previous negativity. The computer selected test responses within a window (±10 msec) of the conditioning stimulus response latency
(37). P50 gating ratios were calculated as (test stimulus response/conditioning stimulus response) × 100. Suppression ([conditioning stimulus response minus test stimulus response]/test stimulus response) was also calculated for comparison with other reports
(12).
Statistical Analysis
For comparing demographic data between the schizophrenia patients and healthy subjects, the chi-square test was used wherever appropriate. Since there were no significant age or gender effects on the evoked potential parameters (amplitude, latency, or gating ratio), these variables were not included in analyses as covariates and will not be reported on further. Student’s t tests were used to compare healthy subjects and schizophrenia patients. ANOVA was used for comparing the differences in each parameter between healthy subjects and 1) typical and atypical neuroleptic treatment groups and 2) specific medication groups. Post hoc tests with Bonferroni corrections for multiple comparisons were used to determine which pairs of means differed significantly.
Results
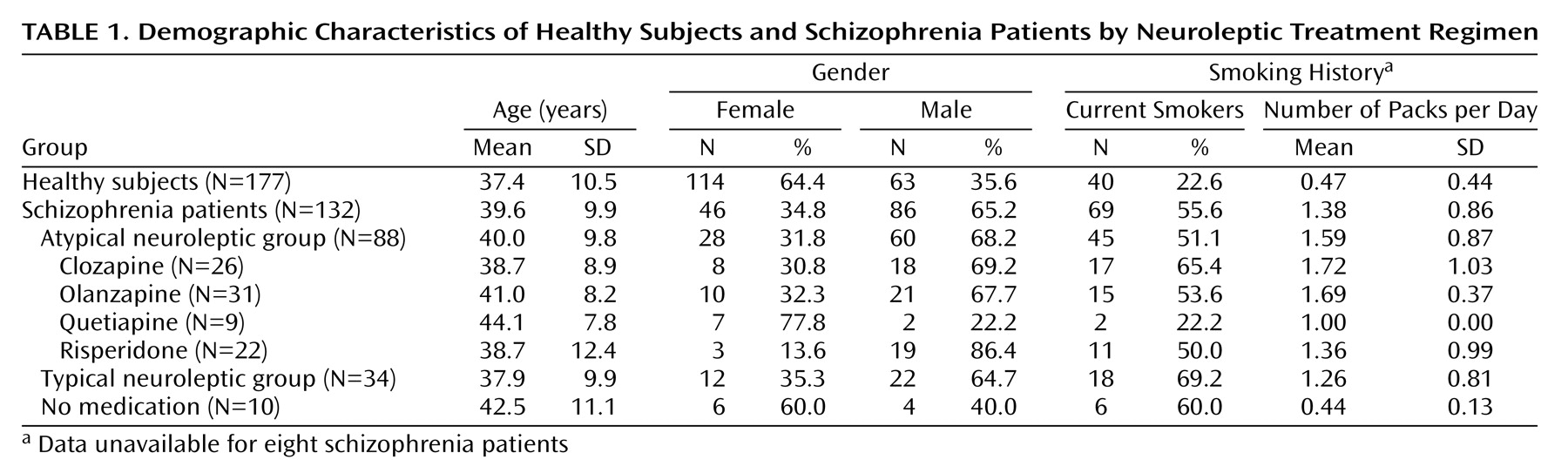
Schizophrenia patients had significantly more impaired P50 sensory gating than did healthy subjects. Typical recordings are shown in
Figure 1. Ninety-one percent of healthy subjects had P50 ratios that were within the previously established normal range compared with 23% of the schizophrenia patients. Only about 10% of patients receiving typical or no medications had P50 sensory gating ratios in the normal range, similar to previously reported figures
(16). There were no significant differences in gating between patients treated with different classes of typical neuroleptics.
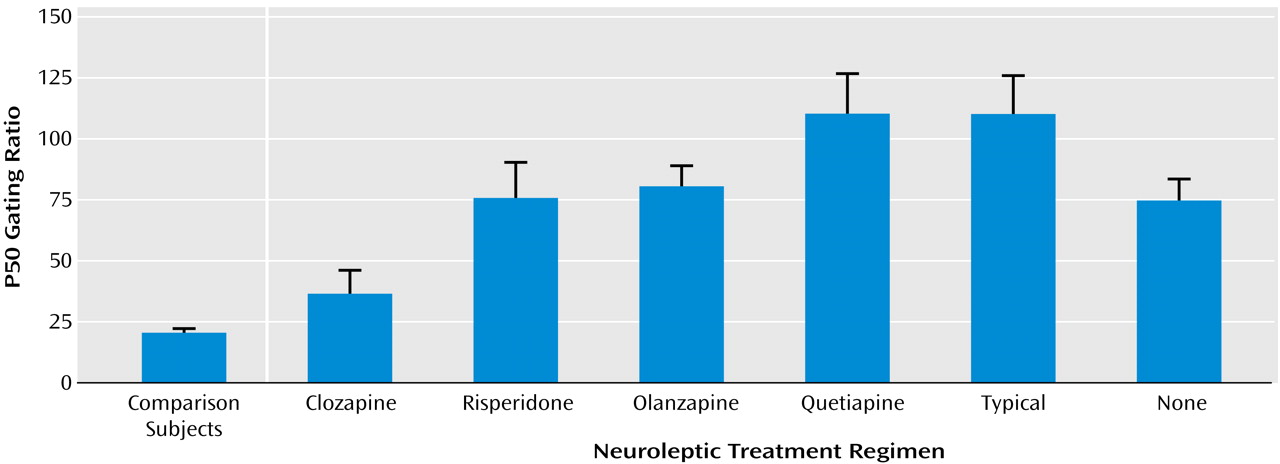
In contrast, 30% of patients treated with various atypical neuroleptics had ratios within the normal range. There were significant differences in P50 sensory gating between the different atypical drugs (
Figure 2). More patients taking clozapine had P50 ratios under 40% than did any other group. Furthermore, only the clozapine group had a mean gating ratio that was not significantly different from the healthy subjects. Using a Bonferroni adjustment for pairwise comparisons, patients treated with clozapine had significantly better sensory gating ratios than patients receiving any of the other atypical medications (clozapine versus risperidone: p<0.04; clozapine versus olanzapine: p=0.009; clozapine versus quetiapine: p=0.001). The mean P50 ratios of the other groups were not statistically different from one another (
Figure 2). Sixty-two percent of patients treated with clozapine had P50 ratios within the normal range, compared with 24% of patients receiving risperidone, 14% of patients receiving olanzapine, and 0.0% of patients receiving quetiapine (χ
2=23.5, df=3, p=0.001).
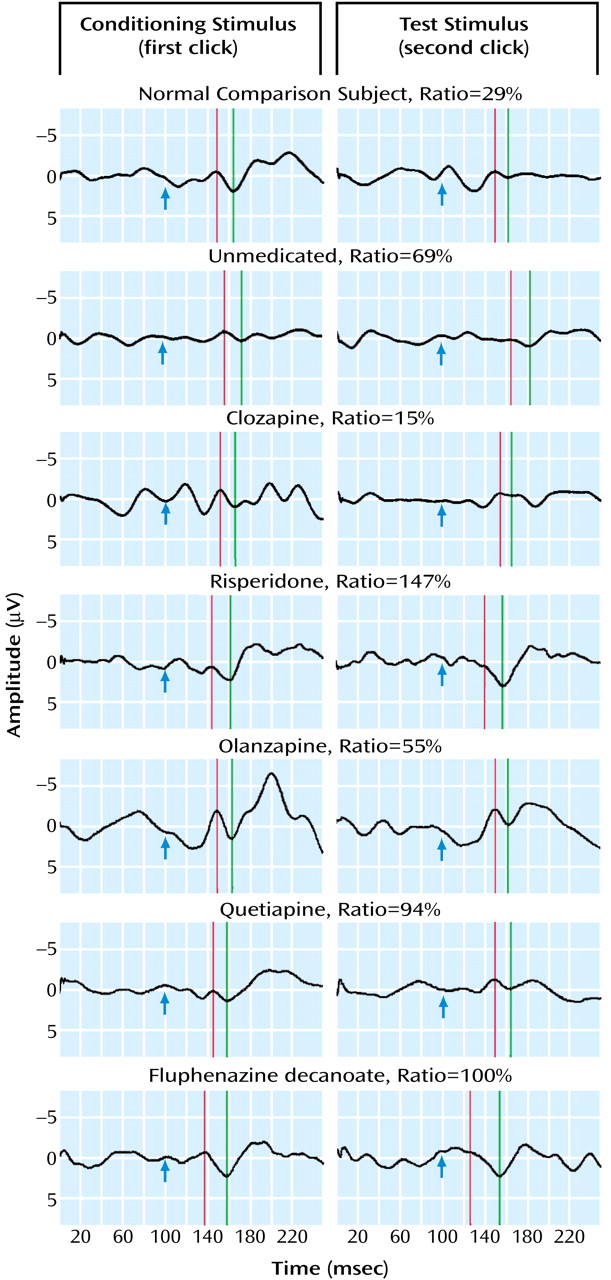
As seen in
Table 2, patients treated with clozapine had a mean P50 gating ratio that was not significantly higher than healthy subjects (mean=36.7 [SD=44.2] and 19.8 [SD=21.0], respectively). All patients receiving typical and atypical neuroleptics had significantly higher (i.e., poorer) P50 gating ratios than both healthy subjects and patients treated with clozapine. Patients treated with risperidone had gating ratios that were significantly better than patients receiving typical neuroleptics (p<0.03). Neither olanzapine nor quetiapine had effects on sensory gating that were different from either typical neuroleptics or no treatment.
The conditioning amplitudes were also significantly different across the groups (
Table 2). Post hoc comparisons show that patients treated with olanzapine had significantly smaller amplitudes than healthy subjects (p=0.01). There were no other significant differences in conditioning amplitude between any of the other groups.
There was a small but significant correlation between the amplitude of the conditioning response and sensory gating in the schizophrenia patients (r=–0.20, df=130, p<0.001), accounting for 4% of the variance in their gating. This result replicates the findings reported in Adler et al.
(4). The healthy group had a correlation of only –0.016.
There were no significant differences between any of the groups on the latency of the conditioning P50 waves.
A comparison of Positive and Negative Symptom Scale scores in patients treated with atypical medications other than clozapine suggests that there were no significant differences in clinical functioning between the groups receiving risperidone, quetiapine, or olanzapine. The positive scores ranged from 7 to 41, with a mean of 16.7 (SD=6, median=16.0). The negative scores ranged from 7 to 30, with a mean of 16.8 (SD=6.4, median=16.0). There were no significant differences between male and female subjects on any of the Positive and Negative Symptom Scale scores, and no significant relationship with age or with any of the electrophysiological measures.
Smoking history was obtained from 301 subjects: 109 were current smokers, 192 were not (
Table 1). Significantly more schizophrenia subjects (55.6%) smoked than healthy subjects (22.6%) (χ
2=35.27, df=2, p<0.001). There was no significant difference in the percentages of schizophrenia smokers in each of the medication groups. In the healthy subjects, there were no differences in any electrophysiological measures between smokers and nonsmokers. Schizophrenia patients taking atypical medications who smoked had significantly lower P50 ratios than those who did not (mean=55.4 [SD=49.8] versus 83.2 [SD=53.5], respectively; t=2.5, df=86, p<0.02). Further investigation of this finding showed that this was entirely due to the differences between smokers and nonsmokers taking clozapine (mean=23.1 [SD=32.2] versus 62.4 [SD=53.7]; t=2.3, df=24, p<0.03). There were no differences between P50 latency and amplitude of schizophrenia smokers as a group or in any of the subgroupings. When corrected for multiple comparisons, there were no significant correlations between the number of packs smoked per day and P50 gating, amplitude, or latency overall or in any of the groups.
Discussion
The aim of this study was to investigate the relationship between sensory gating of the auditory evoked potential P50 wave and treatment with typical and atypical neuroleptics. Consistent with previous studies
(9–
12,
38), schizophrenia patients had, as a group, significantly poorer sensory gating than healthy subjects. Patients treated with the newer atypical medications had gating that fell between the healthy subjects and patients receiving typical neuroleptics.
Subjects receiving typical neuroleptics did not differ significantly from one another in their gating nor in the amplitude and latency of the P50 wave. Subjects receiving atypical medications did significantly differ from one another—some falling within the normal range of gating, others exhibiting gating that was significantly abnormal—despite clinical ratings that were very similar. These findings are consistent with reports by Light et al.
(24) of improvement of sensory gating with treatment by atypical medications.
Of the antipsychotic medications tested, only clozapine treatment resulted in a mean P50 sensory gating ratio in the normal range. Subjects receiving clozapine had significantly better P50 sensory gating than subjects in any of the other treatment groups and were not statistically distinguishable from healthy subjects. The other typical and atypical medications did not differ from one another except for a significant difference between patients receiving olanzapine and those receiving typical medications. Subjects receiving olanzapine, however, continued to have significantly more impaired P50 sensory gating than healthy subjects and patients treated with clozapine. These findings suggest that atypical medications as a whole have some impact on neurophysiological functioning but that clozapine shows a unique significant advantage. This difference may be similar to the differing efficacy of the atypical medications on clinical functioning.
Since this study was cross-sectional and not longitudinal, it could be argued that subjects had been non-randomly assigned to treatment medications and that the psychophysiological differences evident in this study were a reflection of underlying differences that led to the selection of treatment. Longitudinal studies of patients initiating clozapine treatment, however, show that sensory gating does normalize with this specific treatment in concert with clinical improvement
(22). Most subjects reported here had been treated with multiple medications, and nonresponse to several was a precursor to treatment with clozapine. This requirement suggests that the group treated with clozapine is, a priori, the most treatment-resistant group and therefore not the most likely group to have normal sensory gating. However, a definitive answer to this question would require studying the same subjects treated with a variety of neuroleptic medications, both typical and atypical.
Clozapine’s apparently enhanced effects on clinical symptoms and neurocognition have prompted numerous inquiries into how its mechanism of action differs from those of older first-generation and the newer atypical medications. The hypothesis that enhanced serotonin 5-HT
2A and diminished dopamine D
2 receptor activity are what convey atypical antipsychotic effects has resulted in the synthesis of a number of drugs, including risperidone, olanzapine, and quetiapine, that are successful antipsychotic drugs, with significantly lower extrapyramidal effects than drugs such as haloperidol
(39). However, these newer atypical drugs do not appear to capture all of clozapine’s enhanced clinical effect
(40). In this study, clozapine also uniquely normalized gating of P50 responses in the paired auditory stimulus paradigm. The neuronal mechanisms involved in this inhibitory phenomenon might therefore be examined as possible candidate mechanisms that differentiate clozapine from other atypical antipsychotics.
The mechanism of P50 inhibition is itself complex, but a critical element appears to be cholinergic stimulation of inhibitory interneurons via α7 nicotinic acetylcholine receptors. In schizophrenia, the loss of inhibition of P50 responses has been genetically linked to the locus of this gene for this receptor
(41). Clozapine itself is not known to bind to α7 receptors. However, it increases the release of acetylcholine in the hippocampus, one of the sites of generation of P50 and a site that contains α7 nicotinic receptors in abundance
(42). Therefore, it could have an indirect effect on the α7 nicotinic receptor. Direct agonists of the receptor, such as nicotine, also normalize P50 inhibition in schizophrenia
(43,
44). Furthermore, in animal models, selective blockade of α7 nicotinic receptors prevents P50 from normalizing inhibition of hippocampal auditory evoked responses in the paired auditory stimulus paradigm
(45). In schizophrenia, treatment with clozapine, but not other atypical antipsychotics or older neuroleptics, diminishes smoking behavior
(46). Thus, several pieces of evidence point to a possible effect of clozapine on cholinergic neurotransmission. The underlying mechanism of this effect of clozapine is not known, but it could involve serotonin 5-HT
3 receptors, which normally inhibit acetylcholine release in the hippocampus
(47). We have shown that the 5-HT
3 receptor antagonist ondanseron also normalizes P50 inhibition in schizophrenia
(48). If serotonergic control of cholinergic activation of inhibitory interneurons is one of the mechanisms of action of clozapine, it is likely to be only one aspect of the full antipsychotic effect, but perhaps one that is currently not being adequately addressed by other atypical antipsychotics.