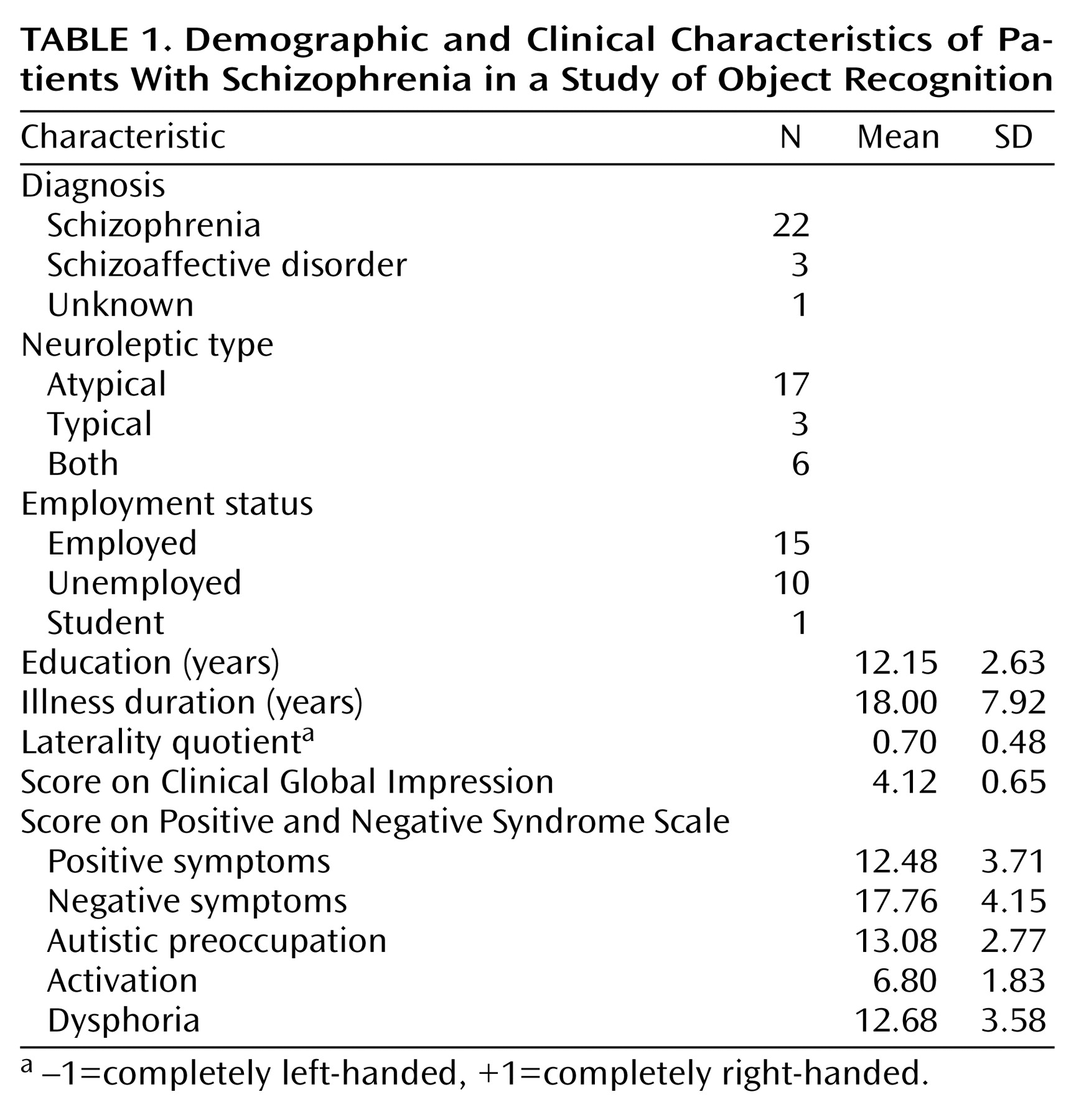
Perceptual closure refers to the ability of the brain to form complete object representations on the basis of fragmentary visual information
(6,
9). It is assumed that the brain automatically “fills in” missing object information, which is consistent with results of single-cell studies of the primate visual cortex
(10–
12). Investigation of perceptual closure in patients with schizophrenia provides an index of their ability to form complete representations on the basis of partial information. The phenomenon of perceptual closure also represents an ideal domain within which to examine the dependence of sensory processing dysfunction on top-down regulation in patients with schizophrenia. Although the ability to “close” an image is primarily tied to the amount of visual information provided, the level of information required for object recognition can be manipulated
(6,
8). Thus, for example, if an image is repeated either immediately or after some delay, participants are able to make an identification with significantly less visual information (i.e., at significantly greater levels of stimulus fragmentation). Also, if participants are given a word prompt that potentially names the object, less visual information is required for recognition when the prompt names the object pictured than when it does not. Repetition priming assesses the ability of the visual system to maintain stimulus representations over time, while word prompting assesses the use of nonfigural information to aid figural interpretation. For word prompting to occur, visual regions must receive top-down information flow from language regions and other areas of the cortex
(13,
14). The present study assesses the integrity of modulatory influences on perceptual closure in patients with schizophrenia, along with the integrity of perceptual closure itself.
Perceptual closure is subserved primarily by the lateral occipital complex
(9,
15,
16), which lies at the height of the ventral visual-stream object-processing hierarchy (the “what” stream)
(15,
17). Presentation of identifiable objects in normal subjects leads to focal bilateral lateral occipital activation
(18,
19). Moreover, object recognition in circumstances of partial information is associated with the generation of an event-related potential component that we termed “N
cl,” which is localized over the lateral occipital complex
(9). Given that perceptual closure has a specific neural correlate, compromised integrity of object recognition in patients with schizophrenia implicates the structures of the lateral occipital complex that generate it. Thus, the present study permits analysis of information processing in patients with schizophrenia at the level of the visual sensory cortex.
Results
This study consisted of three parts. Part 1 tested the participants’ ability to identify the objects in partially fragmented pictures to which they had no previous exposure. The level of fragmentation at which correct object recognition occurred (identification threshold, referred to here as “the level of identification”) was treated as the primary dependent variable. Parts 2 and 3 of the testing evaluated the degree to which the identification threshold during the test phase was modulated by preexposure to the complete pictures. Part 3 also tested the effect of word prompting on object recognition. Higher numbers of images reflected more fragmentation; thus a higher number at object identification indicated better performance. When object recognition occurred during the test phases, both the patients with schizophrenia and the normal comparison subjects demonstrated accuracy above 93%; there was no significant difference in performance between the groups.
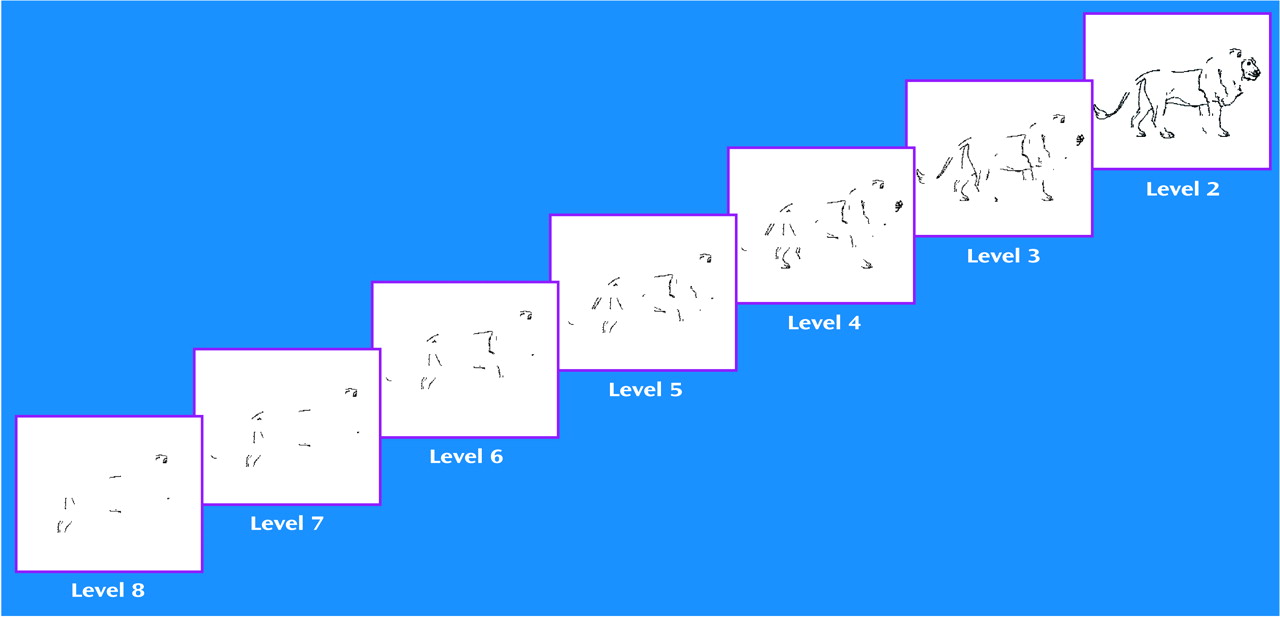
In part 1, data were analyzed by using a one-way, between-group ANOVA. The patients with schizophrenia showed significant impairment in object recognition, as evidenced by the necessity for a more complete image to appear before object recognition was possible (higher threshold of identification;
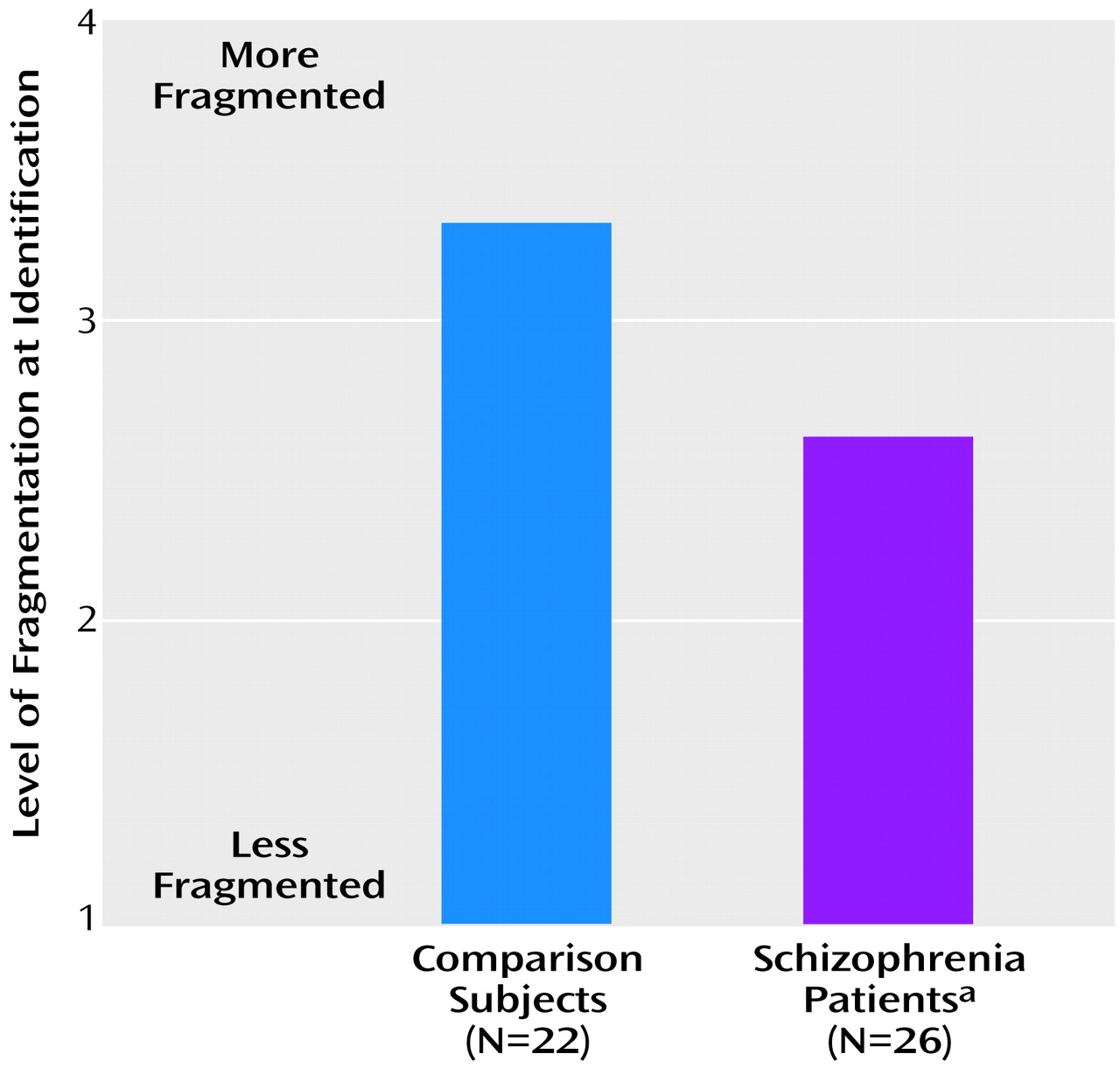
Figure 2). Similarly, when the proportions of pictures recognized at each level by the two groups were analyzed, there was a rightward shift in the psychometric function for schizophrenic patients in relation to the comparison subjects (
Figure 3). Slopes for the two groups, however, were similar.
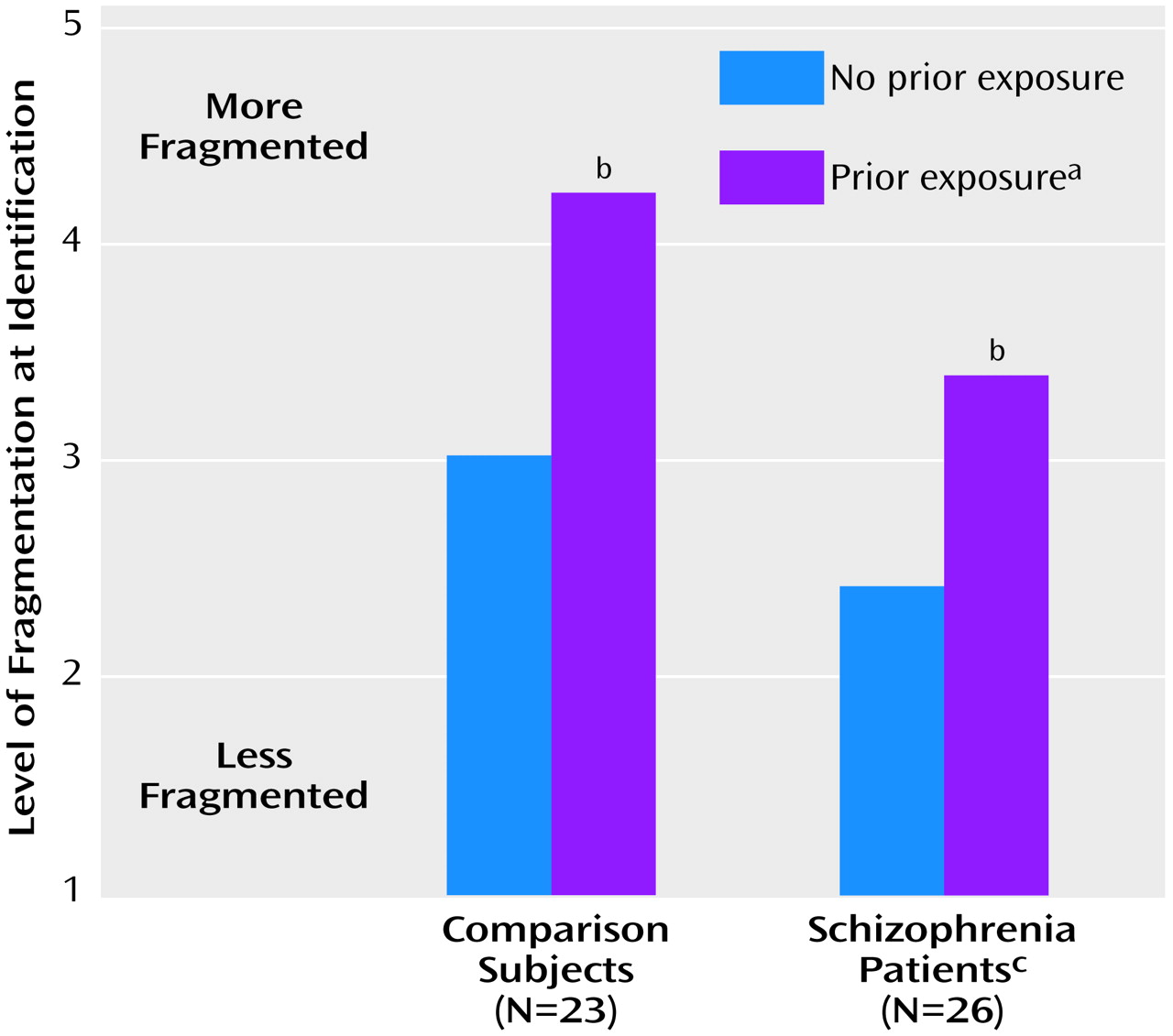
In part 2, data were analyzed by using a two-way (group-by-repetition) ANOVA, with repetition coded as a within-group factor. Both schizophrenic patients and nonpsychiatric comparison participants experienced the expected repetition priming effect, as evidenced by a significant shift in object-recognition threshold (level of identification) as a function of previous exposure (
Figure 4). As in part 1, schizophrenic patients required significantly more complete pictures than the comparison subjects before they were able to identify the objects, whether or not the pictures had been presented previously. However, the schizophrenia patients benefited as much as the comparison subjects from previous exposure to the complete pictures, as reflected by a significant improvement within both groups (comparison subjects: t=10.28, df=22, p<0.001; patients: t=11.06, df=25, p<0.001) and a nonsignificant group-by-repetition interaction (F=2.4, df=1, 47, p=0.13).
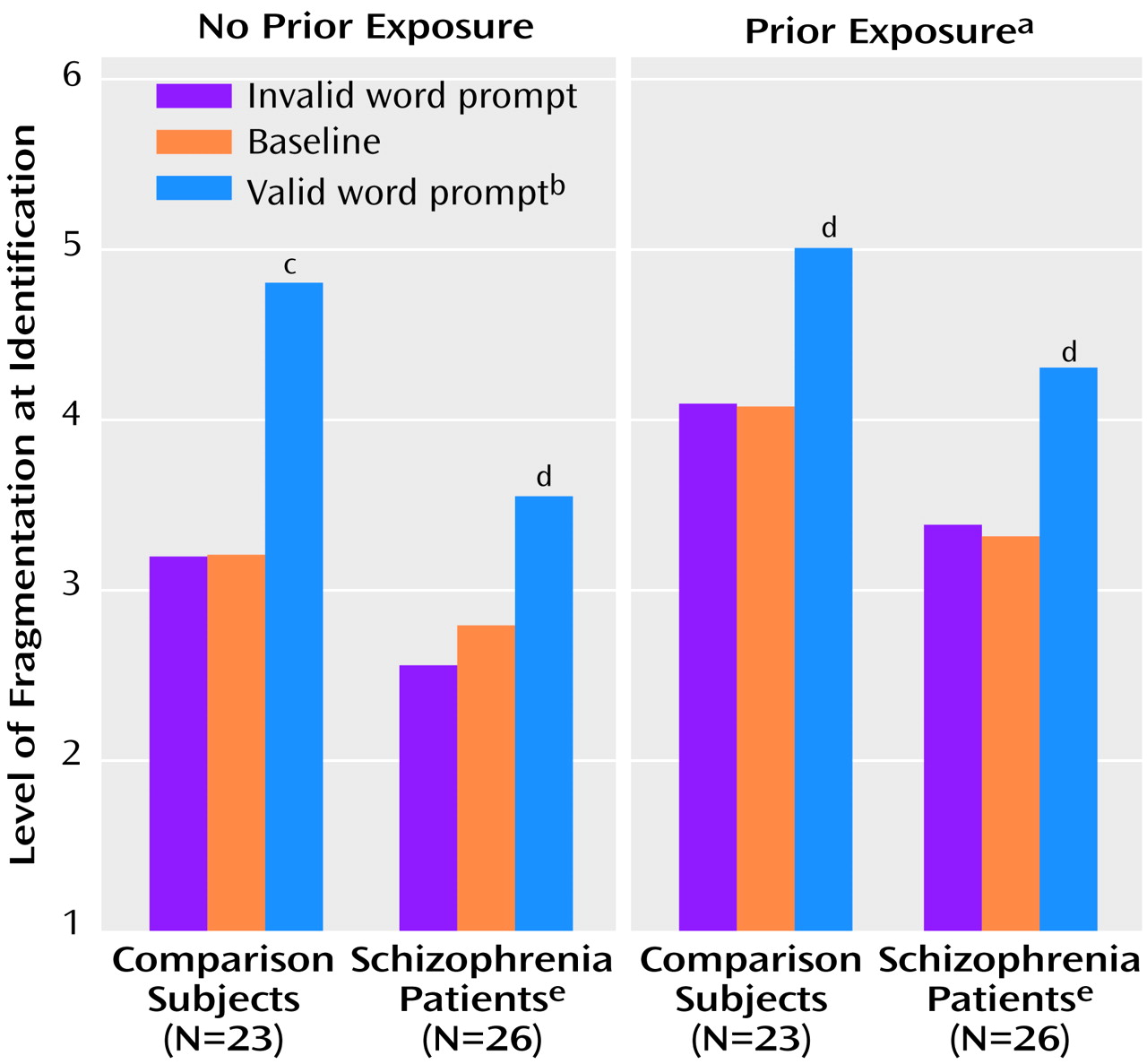
In part 3, the combined effects of repetition priming and word prompting were assessed in a three-way (group-by-repetition-by-prompt type) ANOVA. Repetition and prompt type were coded as within-group factors. Prior exposure and word prompting contributed independently to performance, as reflected by significant main effects of repetition and prompt type (
Figure 5) and the lack of a significant repetition-by-prompt-type interaction (F=1.9, df=2, 94, p=0.16). As in parts 1 and 2 of testing, the schizophrenic participants were highly impaired in their ability to identify fragmented pictures of objects across conditions, as reflected in a significantly higher threshold level of identification (
Figure 5). Significant, similar effects in magnitude of word prompting were observed for the schizophrenia patients and the comparison subjects in both conditions of no prior exposure (comparison subjects: t=6.91, df=22, p<0.001; schizophrenia patients: t=4.71, df=25, p<0.001) and prior exposure (comparison subjects: t=3.21, df=22, p<0.005; schizophrenia patients: t=4.72, df=25, p<0.001). Furthermore, neither the group-by-repetition (F=0.1, df=1, 47, p=0.80) nor the group-by-prompt-type (F=1.6, df=2, 94, p=0.22) interaction effects were significant, indicating that the patients with schizophrenia benefited as much as the comparison subjects from both prior experience and having seen valid word prompts.
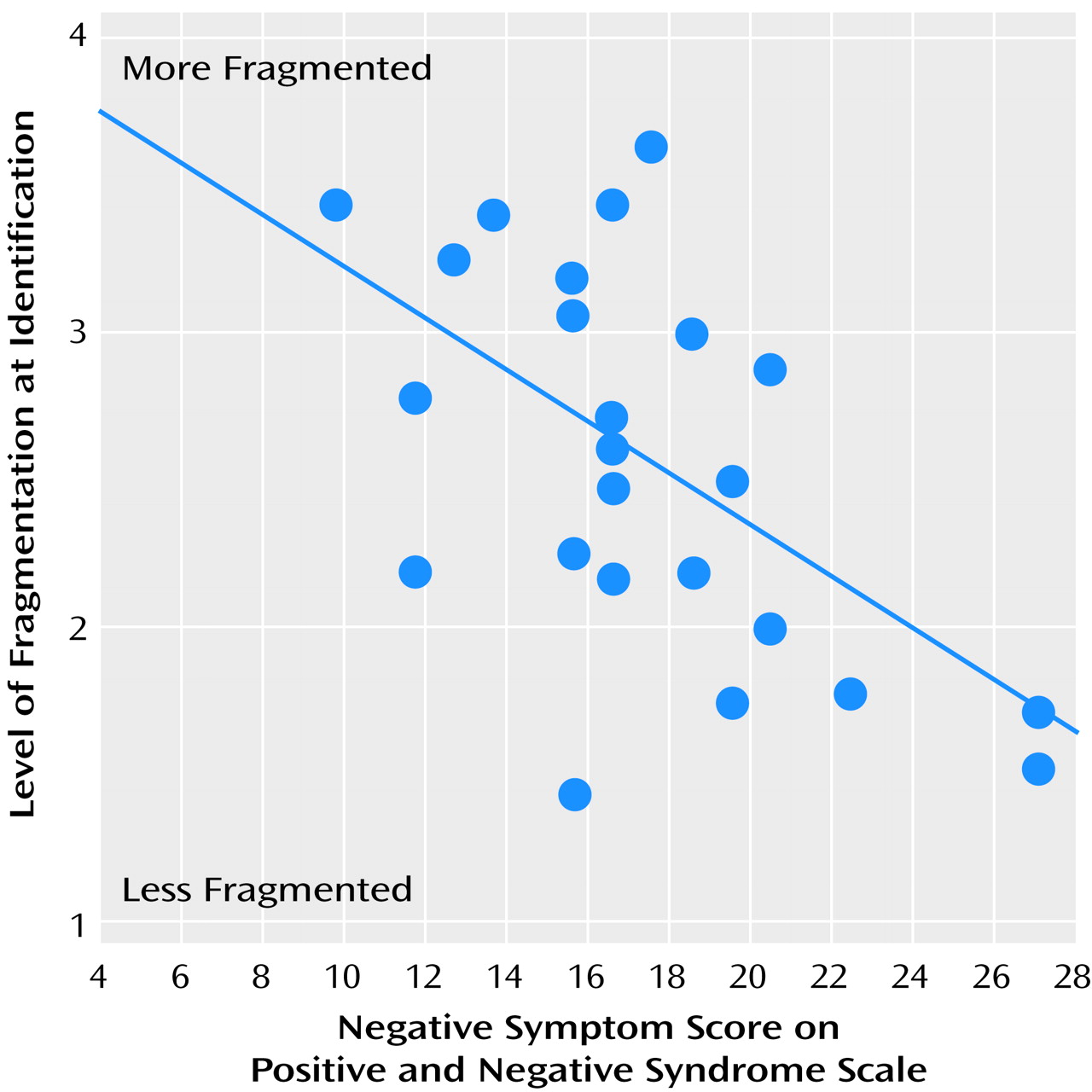
A significant positive correlation was found between level of identification, as measured in part 1 of the testing, and negative symptom score on the Positive and Negative Syndrome Scale (r=–0.54, p=0.005) (
Figure 6). Similar correlations were found for results from parts 2 and 3 of the testing. The level of identification did not correlate with positive symptom or autistic preoccupation subscale scores. Mean IQs for the patient and comparison groups were 98.7 and 110.4, respectively. Although there was a between-group difference in IQ (t=–3.73, df=46, p=0.001), the between-group difference in level of identification, as measured in part 1 of the testing, remained significant after covariation for IQ (F=14.5, df=1, 44. p<0.001). Similar significant differences remained after covariation for results from parts 2 and 3 of the testing.
Discussion
The term “perceptual closure” refers to the collection of neural processes that permits object recognition to occur when only partial visual information is present. Physiologic evidence suggests that these processes may involve interpolation, or “filling in,” of the missing information
(12). The phenomenon of perceptual closure has been extensively studied with the use of fragmented images, both behaviorally
(6–
8) and electrophysiologically
(9,
16), in normal subjects, but, to our knowledge, it has not been evaluated previously in subjects with schizophrenia. This study provides the first demonstration that perceptual closure is impaired in patients with schizophrenia, leading to deficits in object recognition. Nonetheless, patients derive benefit comparable to that of comparison subjects from prior exposure to complete pictures and from having seen valid word prompts that name the object subsequently presented in a fragmented picture, suggesting intact top-down regulation.
Processing of object forms occurs primarily within the ventral visual stream
(26), particularly in the lateral occipital complex
(27). Impaired object recognition in patients with schizophrenia, as reflected in this study, would thus be consistent with dysfunction within ventral visual-stream sensory regions and especially in the lateral occipital complex. Lower cerebral blood flow has been observed in the vicinity of the lateral occipital complex in schizophrenia patients
(5), supporting the notion that deficits in perceptual closure reflect impaired local processing within visual brain regions. Furthermore, because the inferior temporal area in the monkey, which is likely homologous to the lateral occipital complex in humans, plays a critical role in visual backward masking
(28), it is possible that dysfunction in the lateral occipital complex could also account for the well-described visual backward-masking deficits associated with schizophrenia
(29–
31). In our previous high-density electrical mapping studies
(9,
16), we identified a novel event-related potential component—N
cl—that reflects an incremental increase in activity in object-recognition areas of the lateral occipital complex during the presentation of fragmented pictures of objects by means of the ascending method of limits. We proposed that N
cl reflects the processes of perceptual closure, in which the brain attempts to “fill in” the missing pieces of an object until recognition is achieved. It is these neural processes that we believe to be critical for performance in the present study, but they appear to be unaffected by priming or prompting manipulations. This conclusion is supported by our work showing an “early” electrophysiologic index of repetition priming in a perceptual closure task
(16) and other work showing “late” indices of semantic priming
(32).
In addition to demonstrating a deficit in visual sensory processing, the present study also provides evidence that the deficit is not attributable to top-down dysregulation of structures in the ventral visual stream. Two manipulations have been shown to affect the level of sensory information needed to successfully identify an object. First, if an object has been shown previously in identifiable form (either as complete or fragmentary), subsequent recognition is facilitated
(6), as is generation of N
cl (16). Second, if participants are given a prompt that potentially identifies the object pictured, recognition is facilitated
(33; unpublished report by Matsukawa et al.). In the present study, the patients with schizophrenia derived benefit similar to that of comparison subjects from stimulus repetition and word prompting, although across conditions, the patients required more information than the comparison subjects to successfully identify an object. Thus, the study shows a clear differential deficit whereby patients were impaired in the sensory aspects of the task but were relatively unimpaired with regard to higher-order functions.
The pattern of impairment observed in this study differs from that of other neuropsychiatric disorders. Thus, although Alzheimer’s disease has been associated with deficits in object recognition, these are accompanied by profound deficits in repetition priming
(34–
37). In contrast, subjects with Huntington’s disease may show enhanced priming
(37). Finally, subjects with Parkinson’s disease have not shown deficits in object recognition or priming
(35). Thus, the present pattern may be specific to subjects with schizophrenia.
A study by Newcombe and Russell
(38) revealed that patients with temporal damage show poor performance on a “closure” task that requires age and gender judgments, supporting localization of perceptual closure to the lateral occipital complex. Patients with right parietal lesions have been shown to have a syndrome termed “apperceptive agnosia”
(39) characterized by impaired shape perception and suggesting that impaired parietal input to the lateral occipital cortex may contribute to the observed deficit
(40,
41). In contrast, patients with predominant frontocortical lesions showed more robust deficits in priming than in object recognition itself, supporting the notion that the present pattern of impairment cannot be attributed to prefrontal dysfunction
(42).
The present findings are consistent with deficits demonstrated in patients with schizophrenia in a variety of perceptual organization tasks
(43–
45). However, an advantage of the present tasks is that “perceptual closure” is a highly specific term that refers to a distinct set of processes that underlie the identification of objects in fragmented pictures, such as those in the present study. The current study sets the stage for future electrophysiological studies of perceptual closure in patients with schizophrenia, in which the neural processes underlying these behavioral effects can be examined directly. Our findings are also consistent with studies showing intact repetition priming and implicit learning in subjects with schizophrenia
(46,
47). Again, implicit learning in the context of a perceptual closure task has a well-defined electrophysiological signature
(16), whereas such is not the case with other forms of implicit learning.
This is the first study of which we are aware of object recognition in patients with schizophrenia. However, at least one prior study investigated the phenomenon of perceptual closure
(48). In that study, patients were asked to reproduce line drawings with gaps. Comparison subjects automatically filled in the gaps because of the operation of perceptual closure, drawing more complete pictures of objects than had been requested. In contrast, the patients did not fill in the gaps; thus, paradoxically, they drew pictures that better approximated the fragmented originals than did the comparison subjects. Since the time of this 1961 study, little work has been done on the impairment of perceptual closure in patients with schizophrenia. The present study builds on earlier work and offers an improved method for quantifying the perceptual closure deficit.
It has been appreciated for at least 30 years that schizophrenic patients perform poorly on a wide variety of cognitive tasks. To some extent, this observation has been used to suggest that individual deficits are significant only to the degree that they stand out from a background of widespread dysfunction
(49). More recently, however, it has been accepted that a widespread, multifocal deficit is, of itself, a crucial aspect of schizophrenia and a primary predictor of poor outcome
(3,
4). The present study suggests that such deficits arise from distributed dysfunction within multiple brain regions, involving sensory as well as cognitive areas. These findings are consistent with those of parallel studies that have demonstrated sensory processing deficits within auditory
(50) and somatosensory
(51) brain regions.
There are several indications that the observed deficit in perceptual closure was not due to factors such as impaired cooperation or motivation on the part of the participants. First, with sufficient information, the patients recognized the objects correctly as often as the comparison subjects; both groups correctly identified the objects over 90% of the time. Only correct responses were analyzed. Second, the slopes of the performance curves were very similar for schizophrenia patients and comparison subjects, although the patient curve was shifted significantly to the right (
Figure 3). Thus, similar processes were engaged in both groups, although processing precision in the patient group was less than that of the comparison group. Finally and most important, the patients with schizophrenia were able to derive as much benefit as the comparison subjects from repetition priming and word prompting. Repetition priming and word prompting require at least as much task engagement as object recognition. Overall, therefore, the present study suggests that deficits in sensory-level processing may contribute significantly to widespread cognitive dysfunction in patients with schizophrenia and that such deficits reflect intrinsic dysfunction within sensory brain regions.
Although the present study was designed to assess the integrity of object recognition, a ventral visual-stream process, it should be noted that deficits in visual processing in patients with schizophrenia are not likely confined to this pathway. Deficits in motion detection have also been reported
(52), as have deficits in regulation of smooth-pursuit eye movements
(53). Both sets of deficits are postulated to reflect dysfunction of the dorsal-stream visual pathway. We have recently observed selective deficits in magnocellular processing, suggesting dysfunction even at the level of the lateral geniculate nucleus or extrastriate cortex
(54). Thus, in addition to reflecting dysfunction within the lateral occipital complex, the deficit in perceptual closure may also reflect impaired information processing within earlier sensory regions.
The patients in the present study were recruited from outpatient and inpatient facilities. This permitted recruitment of schizophrenic patients with a wide range of positive, negative, and cognitive symptoms. A significant correlation was found between negative symptoms and level of fragmentation at object recognition. Studies of the information-processing correlates of positive and negative symptoms
(55) have found that patients with negative symptoms have elevated stimulus-recognition thresholds and deficits in the time-dependent processing of information. The present findings are consistent with this earlier work.
Many real-world stimuli to which individuals are exposed are observed in fragmentary form. Perceptual closure permits relatively automatic identification of such objects (e.g., “a cat behind venetian blinds”). The correlation between the deficit in perceptual closure and the negative symptom score suggests that patients with negative symptoms may navigate a world that is form-impoverished and that the absence of salient forms may directly contribute to such diagnostic features as a lack of interest in the environment. The correlation may also reflect operation of a common mediating variable. For example, dysfunction of
N-methyl-
d-aspartate (NMDA) receptors is postulated to play a key role in the mediation of negative symptoms
(56). Furthermore, these receptors contribute significantly to the development of feature-detection circuits within visual sensory regions
(57). NMDA receptor dysfunction could thus contribute to both symptoms and information-processing aspects of the disorder.
A limitation of the present study is that all of the patients with schizophrenia were receiving medication at the time of testing
(58). However, there was no significant correlation between neuroleptic dose and performance. Furthermore, antipsychotic effects would not explain the finding of preserved higher-order processing despite impaired perceptual closure within the patient group. Because dopamine is an important neurotransmitter in the retina
(59,
60), the possibility that endogenous dopamine deficiency contributes to the observed deficit in perceptual closure must be considered
(61). It has been shown, however, that visual acuity is unaffected by the dopamine-blocking antipsychotic haloperidol
(62). Furthermore, the recognition of objects in fragmented pictures has been found to be unimpaired in patients with Parkinson’s disease, which is characterized by a deficiency of dopamine
(35). Hence, it is unlikely that the effects of antipsychotic dopamine blockers can account for the deficit in perceptual closure observed in the present study.